Multiomic profiling reveals metabolic alterations mediating aberrant platelet activity and inflammation in myeloproliferative neoplasms
- PMID: 38060311
- PMCID: PMC10836808
- DOI: 10.1172/JCI172256
Multiomic profiling reveals metabolic alterations mediating aberrant platelet activity and inflammation in myeloproliferative neoplasms
Abstract
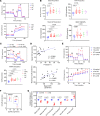
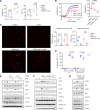
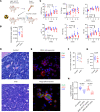
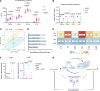
Platelets from patients with myeloproliferative neoplasms (MPNs) exhibit a hyperreactive phenotype. Here, we found elevated P-selectin exposure and platelet-leukocyte aggregates indicating activation of platelets from essential thrombocythemia (ET) patients. Single-cell RNA-seq analysis of primary samples revealed significant enrichment of transcripts related to platelet activation, mTOR, and oxidative phosphorylation in ET patient platelets. These observations were validated via proteomic profiling. Platelet metabolomics revealed distinct metabolic phenotypes consisting of elevated ATP generation accompanied by increases in the levels of multiple intermediates of the tricarboxylic acid cycle, but lower α-ketoglutarate (α-KG) in MPN patients. Inhibition of PI3K/AKT/mTOR signaling significantly reduced metabolic responses and hyperreactivity in MPN patient platelets, while α-KG supplementation markedly reduced oxygen consumption and ATP generation. Ex vivo incubation of platelets from both MPN patients and Jak2 V617F-knockin mice with α-KG supplementation significantly reduced platelet activation responses. Oral α-KG supplementation of Jak2 V617F mice decreased splenomegaly and reduced hematocrit, monocyte, and platelet counts. Finally, α-KG treatment significantly decreased proinflammatory cytokine secretion from MPN CD14+ monocytes. Our results reveal a previously unrecognized metabolic disorder in conjunction with aberrant PI3K/AKT/mTOR signaling that contributes to platelet hyperreactivity in MPN patients.
Keywords: Hematology; Platelets.
Conflict of interest statement
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

