Lung microvascular occlusion by platelet-rich neutrophil-platelet aggregates promotes cigarette smoke-induced severe flu
- PMID: 38060312
- PMCID: PMC10906226
- DOI: 10.1172/jci.insight.167299
Lung microvascular occlusion by platelet-rich neutrophil-platelet aggregates promotes cigarette smoke-induced severe flu
Abstract
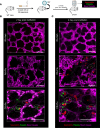
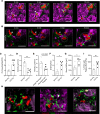
Cigarette smoking is associated with a higher risk of ICU admissions among patients with flu. However, the etiological mechanism by which cigarette smoke (CS) exacerbates flu remains poorly understood. Here, we show that a mild dose of influenza A virus promotes a severe lung injury in mice preexposed to CS but not room air for 4 weeks. Real-time intravital (in vivo) lung imaging revealed that the development of acute severe respiratory dysfunction in CS- and flu-exposed mice was associated with the accumulation of platelet-rich neutrophil-platelet aggregates (NPAs) in the lung microcirculation within 2 days following flu infection. These platelet-rich NPAs formed in situ and grew larger over time to occlude the lung microvasculature, leading to the development of pulmonary ischemia followed by the infiltration of NPAs and vascular leakage into the alveolar air space. These findings suggest, for the first time to our knowledge, that an acute onset of platelet-driven thrombo-inflammatory response in the lung contributes to the development of CS-induced severe flu.
Keywords: Infectious disease; Influenza; Neutrophils; Platelets; Pulmonology.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

