CRISPR screening identifies BET and mTOR inhibitor synergy in cholangiocarcinoma through serine glycine one carbon
- PMID: 38060314
- PMCID: PMC10906219
- DOI: 10.1172/jci.insight.174220
CRISPR screening identifies BET and mTOR inhibitor synergy in cholangiocarcinoma through serine glycine one carbon
Abstract
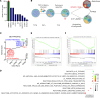
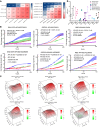
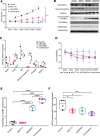
Patients with cholangiocarcinoma have poor clinical outcomes due to late diagnoses, poor prognoses, and limited treatment strategies. To identify drug combinations for this disease, we have conducted a genome-wide CRISPR screen anchored on the bromodomain and extraterminal domain (BET) PROTAC degrader ARV825, from which we identified anticancer synergy when combined with genetic ablation of members of the mTOR pathway. This combination effect was validated using multiple pharmacological BET and mTOR inhibitors, accompanied by increased levels of apoptosis and cell cycle arrest. In a xenograft model, combined BET degradation and mTOR inhibition induced tumor regression. Mechanistically, the 2 inhibitor classes converged on H3K27ac-marked epigenetic suppression of the serine glycine one carbon (SGOC) metabolism pathway, including the key enzymes PHGDH and PSAT1. Knockdown of PSAT1 was sufficient to replicate synergy with single-agent inhibition of either BET or mTOR. Our results tie together epigenetic regulation, metabolism, and apoptosis induction as key therapeutic targets for further exploration in this underserved disease.
Keywords: Cell Biology; Drug screens; Gastroenterology; Liver cancer.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

