Kinetic and thermodynamic control of C(sp2)-H activation enables site-selective borylation
- PMID: 38060669
- PMCID: PMC10898344
- DOI: 10.1126/science.adj6527
Kinetic and thermodynamic control of C(sp2)-H activation enables site-selective borylation
Abstract
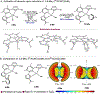
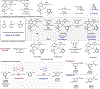
Catalysts that distinguish between electronically distinct carbon-hydrogen (C-H) bonds without relying on steric effects or directing groups are challenging to design. In this work, cobalt precatalysts supported by N-alkyl-imidazole-substituted pyridine dicarbene (ACNC) pincer ligands are described that enable undirected, remote borylation of fluoroaromatics and expansion of scope to include electron-rich arenes, pyridines, and tri- and difluoromethoxylated arenes, thereby addressing one of the major limitations of first-row transition metal C-H functionalization catalysts. Mechanistic studies established a kinetic preference for C-H bond activation at the meta-position despite cobalt-aryl complexes resulting from ortho C-H activation being thermodynamically preferred. Switchable site selectivity in C-H borylation as a function of the boron reagent was thereby preliminarily demonstrated using a single precatalyst.
Conflict of interest statement
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

