Prospective External Validation of the Esbenshade Vanderbilt Models Accurately Predicts Bloodstream Infection Risk in Febrile Non-Neutropenic Children With Cancer
- PMID: 38060973
- PMCID: PMC10906655
- DOI: 10.1200/JCO.23.01814
Prospective External Validation of the Esbenshade Vanderbilt Models Accurately Predicts Bloodstream Infection Risk in Febrile Non-Neutropenic Children With Cancer
Erratum in
-
Erratum: Prospective External Validation of the Esbenshade Vanderbilt Models Accurately Predicts Bloodstream Infection Risk in Febrile Non-Neutropenic Children With Cancer.J Clin Oncol. 2024 Dec 20;42(36):4356. doi: 10.1200/JCO-24-02432. Epub 2024 Nov 8. J Clin Oncol. 2024. PMID: 39514835 No abstract available.
Abstract
Purpose: The optimal management of fever without severe neutropenia (absolute neutrophil count [ANC] ≥500/µL) in pediatric patients with cancer is undefined. The previously proposed Esbenshade Vanderbilt (EsVan) models accurately predict bacterial bloodstream infections (BSIs) in this population and provide risk stratification to aid management, but have lacked prospective external validation.
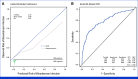
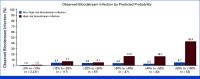
Materials and methods: Episodes of fever with a central venous catheter and ANC ≥500/µL occurring in pediatric patients with cancer were prospectively collected from 18 academic medical centers. Variables included in the EsVan models and 7-day clinical outcomes were collected. Five versions of the EsVan models were applied to the data with calculation of C-statistics for both overall BSI rate and high-risk organism BSI (gram-negative and Staphylococcus aureus BSI), as well as model calibration.
Results: In 2,565 evaluable episodes, the BSI rate was 4.7% (N = 120). Complications for the whole cohort were rare, with 1.1% (N = 27) needing intensive care unit (ICU) care by 7 days, and the all-cause mortality rate was 0.2% (N = 5), with only one potential infection-related death. C-statistics ranged from 0.775 to 0.789 for predicting overall BSI, with improved accuracy in predicting high-risk organism BSI (C-statistic 0.800-0.819). Initial empiric antibiotics were withheld in 14.9% of episodes, with no deaths or ICU admissions attributable to not receiving empiric antibiotics.
Conclusion: The EsVan models, especially EsVan2b, perform very well prospectively across multiple academic medical centers and accurately stratify risk of BSI in episodes of non-neutropenic fever in pediatric patients with cancer. Implementation of routine screening with risk-stratified management for non-neutropenic fever in pediatric patients with cancer could safely reduce unnecessary antibiotic use.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Kelly MJ, Vivier PM, Panken TM, et al. Bacteremia in febrile nonneutropenic pediatric oncology patients. Pediatr Blood Cancer. 2010;54:83–87. - PubMed
-
- Moskalewicz RL, Isenalumhe LL, Luu C, et al. Bacteremia in nonneutropenic pediatric oncology patients with central venous catheters in the ED. Am J Emerg Med. 2017;35:20–24. - PubMed
-
- Walker H, Esbenshade AJ, Dale S, et al. Non-neutropenic fever in children with cancer: Management, outcomes and clinical decision rule validation. Pediatr Blood Cancer. 2022;69:e29931. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

