Deep proteomics reveals incorporation of unedited proteins into mitochondrial protein complexes in Arabidopsis
- PMID: 38060994
- PMCID: PMC11142381
- DOI: 10.1093/plphys/kiad655
Deep proteomics reveals incorporation of unedited proteins into mitochondrial protein complexes in Arabidopsis
Abstract
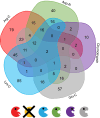
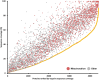
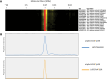
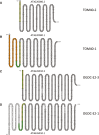
The mitochondrial proteome consists of numerous types of proteins which either are encoded and synthesized in the mitochondria, or encoded in the cell nucleus, synthesized in the cytoplasm and imported into the mitochondria. Their synthesis in the mitochondria, but not in the nucleus, relies on the editing of the primary transcripts of their genes at defined sites. Here, we present an in-depth investigation of the mitochondrial proteome of Arabidopsis (Arabidopsis thaliana) and a public online platform for the exploration of the data. For the analysis of our shotgun proteomic data, an Arabidopsis sequence database was created comprising all available protein sequences from the TAIR10 and Araport11 databases, supplemented with sequences of proteins translated from edited and nonedited transcripts of mitochondria. Amino acid sequences derived from partially edited transcripts were also added to analyze proteins encoded by the mitochondrial genome. Proteins were digested in parallel with six different endoproteases to obtain maximum proteome coverage. The resulting peptide fractions were finally analyzed using liquid chromatography coupled to ion mobility spectrometry and tandem mass spectrometry. We generated a "deep mitochondrial proteome" of 4,692 proteins. 1,339 proteins assigned to mitochondria by the SUBA5 database (https://suba.live) accounted for >80% of the total protein mass of our fractions. The coverage of proteins by identified peptides was particularly high compared to single-protease digests, allowing the exploration of differential splicing and RNA editing events at the protein level. We show that proteins translated from nonedited transcripts can be incorporated into native mitoribosomes and the ATP synthase complex. We present a portal for the use of our data, based on "proteomaps" with directly linked protein data. The portal is available at www.proteomeexplorer.de.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

