Bone turnover change after randomized switch from tenofovir disoproxil to tenofovir alafenamide fumarate in men with HIV
- PMID: 38061030
- PMCID: PMC10906193
- DOI: 10.1097/QAD.0000000000003811
Bone turnover change after randomized switch from tenofovir disoproxil to tenofovir alafenamide fumarate in men with HIV
Abstract
Objective: Bone loss in people with HIV (PWH) is poorly understood. Switching tenofovir disoproxil fumarate (TDF) to tenofovir alafenamide (TAF) has yielded bone mineral density (BMD) increases. PETRAM (NCT#:03405012) investigated whether BMD and bone turnover changes correlate.
Design: Open-label, randomized controlled trial.
Setting: Single-site, outpatient, secondary care.
Participants: Nonosteoporotic, virologically suppressed, cis-male PWH taking TDF/emtricitabine (FTC)/rilpivirine (RPV) for more than 24 weeks.
Intervention: Continuing TDF/FTC/RPV versus switching to TAF/FTC/RPV (1 : 1 randomization).
Main outcome measures: :[ 18 F]NaF-PET/CT for bone turnover (standardized uptake values, SUV mean ) and dual-energy x-ray absorptiometry for lumbar spine and total hip BMD.
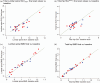
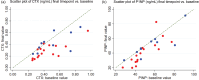
Results: Thirty-two men, median age 51 years, 76% white, median duration TDF/FTC/RPV 49 months, were randomized between 31 August 2018 and 09 March 2020. Sixteen TAF:11 TDF were analyzed. Baseline-final scan range was 23-103 (median 55) weeks. LS-SUV mean decreased for both groups (TAF -7.9% [95% confidence interval -14.4, -1.5], TDF -5.3% [-12.1,1.5], P = 0.57). TH-SUV mean showed minimal changes (TAF +0.3% [-12.2,12.8], TDF +2.9% [-11.1,16.9], P = 0.77). LS-BMD changes were slightly more favorable with TAF but failed to reach significance (TAF +1.7% [0.3,3.1], TDF -0.3 [-1.8,1.2], P = 0.06). Bone turnover markers decreased more with TAF ([CTX -35.3% [-45.7, -24.9], P1NP -17.6% [-26.2, -8.5]) than TDF (-11.6% [-28.8, +5.6] and -6.9% [-19.2, +5.4] respectively); statistical significance was only observed for CTX ( P = 0.02, P1NP, P = 0.17).
Conclusion: Contrary to our hypothesis, lumbar spine and total hip regional bone formation (SUV mean ) and BMD did not differ postswitch to TAF. However, improved LS-BMD and CTX echo other TAF-switch studies. The lack of difference in SUV mean may be due to inadequate power.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Cassetti I. Study 903E Team. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naïve HIV-1-infected patients. HIV Clin Trials 2007; 8:8164–8172. - PubMed
-
- Deeks SG, Overbaugh J, Phillips A, Buchbinder S. HIV infection. Nat Rev Dis Primers 2015; 1:15035. - PubMed
-
- Maggiolo F, Rizzardini G, Raffi F, et al. . Bone mineral density in virologically suppressed people aged 60 years or older with HIV-1 switching from a regimen containing tenofovir disoproxil fumarate to an elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide single-tablet regimen: a multicentre, open-label, phase 3b, randomised trial. Lancet HIV 2019; 6:e655–e666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical