Effects of empagliflozin on progression of chronic kidney disease: a prespecified secondary analysis from the empa-kidney trial
- PMID: 38061371
- PMCID: PMC7615591
- DOI: 10.1016/S2213-8587(23)00321-2
Effects of empagliflozin on progression of chronic kidney disease: a prespecified secondary analysis from the empa-kidney trial
Erratum in
-
Correction to Lancet Diabetes Endocrinol 2024; 12: 39-50.Lancet Diabetes Endocrinol. 2024 Mar;12(3):e16. doi: 10.1016/S2213-8587(24)00033-0. Epub 2024 Jan 29. Lancet Diabetes Endocrinol. 2024. PMID: 38301677 No abstract available.
-
Correction to Lancet Diabetes Endocrinol 2024; 12: 39-50.Lancet Diabetes Endocrinol. 2024 Nov;12(11):e20. doi: 10.1016/S2213-8587(24)00288-2. Epub 2024 Sep 13. Lancet Diabetes Endocrinol. 2024. PMID: 39284330 No abstract available.
Abstract
Background: Sodium-glucose co-transporter-2 (SGLT2) inhibitors reduce progression of chronic kidney disease and the risk of cardiovascular morbidity and mortality in a wide range of patients. However, their effects on kidney disease progression in some patients with chronic kidney disease are unclear because few clinical kidney outcomes occurred among such patients in the completed trials. In particular, some guidelines stratify their level of recommendation about who should be treated with SGLT2 inhibitors based on diabetes status and albuminuria. We aimed to assess the effects of empagliflozin on progression of chronic kidney disease both overall and among specific types of participants in the EMPA-KIDNEY trial.
Methods: EMPA-KIDNEY, a randomised, controlled, phase 3 trial, was conducted at 241 centres in eight countries (Canada, China, Germany, Italy, Japan, Malaysia, the UK, and the USA), and included individuals aged 18 years or older with an estimated glomerular filtration rate (eGFR) of 20 to less than 45 mL/min per 1·73 m2, or with an eGFR of 45 to less than 90 mL/min per 1·73 m2 with a urinary albumin-to-creatinine ratio (uACR) of 200 mg/g or higher. We explored the effects of 10 mg oral empagliflozin once daily versus placebo on the annualised rate of change in estimated glomerular filtration rate (eGFR slope), a tertiary outcome. We studied the acute slope (from randomisation to 2 months) and chronic slope (from 2 months onwards) separately, using shared parameter models to estimate the latter. Analyses were done in all randomly assigned participants by intention to treat. EMPA-KIDNEY is registered at ClinicalTrials.gov, NCT03594110.
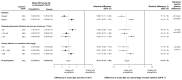
Findings: Between May 15, 2019, and April 16, 2021, 6609 participants were randomly assigned and then followed up for a median of 2·0 years (IQR 1·5-2·4). Prespecified subgroups of eGFR included 2282 (34·5%) participants with an eGFR of less than 30 mL/min per 1·73 m2, 2928 (44·3%) with an eGFR of 30 to less than 45 mL/min per 1·73 m2, and 1399 (21·2%) with an eGFR 45 mL/min per 1·73 m2 or higher. Prespecified subgroups of uACR included 1328 (20·1%) with a uACR of less than 30 mg/g, 1864 (28·2%) with a uACR of 30 to 300 mg/g, and 3417 (51·7%) with a uACR of more than 300 mg/g. Overall, allocation to empagliflozin caused an acute 2·12 mL/min per 1·73 m2 (95% CI 1·83-2·41) reduction in eGFR, equivalent to a 6% (5-6) dip in the first 2 months. After this, it halved the chronic slope from -2·75 to -1·37 mL/min per 1·73 m2 per year (relative difference 50%, 95% CI 42-58). The absolute and relative benefits of empagliflozin on the magnitude of the chronic slope varied significantly depending on diabetes status and baseline levels of eGFR and uACR. In particular, the absolute difference in chronic slopes was lower in patients with lower baseline uACR, but because this group progressed more slowly than those with higher uACR, this translated to a larger relative difference in chronic slopes in this group (86% [36-136] reduction in the chronic slope among those with baseline uACR <30 mg/g compared with a 29% [19-38] reduction for those with baseline uACR ≥2000 mg/g; ptrend<0·0001).
Interpretation: Empagliflozin slowed the rate of progression of chronic kidney disease among all types of participant in the EMPA-KIDNEY trial, including those with little albuminuria. Albuminuria alone should not be used to determine whether to treat with an SGLT2 inhibitor.
Funding: Boehringer Ingelheim and Eli Lilly.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declarations of interests The Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford (Oxford, UK), has a staff policy of not accepting honoraria or other payments from the pharmaceutical industry, except for the reimbursement of costs to participate in scientific meetings. NS, RH, PKJ, JRE, DP, KJM, SYAN, ES, DZ, MH, WS, KW, MJL, CB, and WGH report grant funding paid to their institution from Boehringer Ingelheim and Eli Lilly, and funding from the UK Medical Research Council (MRC) to the CTSU (reference number, MC_UU_00017/3), the British Heart Foundation, the UK National Institute for Health and Care Research (NIHR) Biomedical Research Council, and Health Data Research (UK). NS additionally acknowledges grant funding paid to their institution from Novo Nordisk. RH additionally acknowledges provision of investigational medicinal products for clinical trials from Roche, GSK/Vir, and Combiphar. DP additionally acknowledges grant funding paid to their institution from Novartis and Novo Nordisk. ES additionally acknowledges grant funding paid to their institution from Merck. MH additionally acknowledges grant funding paid to their institution from Novartis. MJL additionally acknowledges grant funding paid to their institution from Novartis and Janssen, and donation of treatment for clinical trials from Regeneron and Roche. CB additionally acknowledges roles on monitoring or advisory boards for Merck, NIHR, and the British Heart Foundation; and leadership or fiduciary roles in other boards, societies, committees, or advocacy groups for the European Society of Cardiology and NIHR. WGH was additionally funded by an MRC Kidney Research UK Professor David Kerr Clinician Scientist Award (MR/R007764/1), and acknowledges leadership or fiduciary roles in other boards, societies, committees, or advocacy groups with the European Society of Cardiology, UK Kidney Association, and European Renal Association. CW reports institutional grant funding from Boehringer Ingelheim and Sanofi; consulting fees from Bayer, Boehringer Ingelheim, AstraZeneca, and Astellas; and honoraria for lectures from Bayer, Boehringer Ingelheim, AstraZeneca, Amgen, Sanofi, MSD, Fresenius Medical Care, CSL Vifor, Novartis, and Novo Nordisk. JBG reports grant funding from Boehringer Ingelheim, Merck, Roche, Lilly, and Sanofi/Lexicon; and consulting fees from AstraZeneca, Novo Nordisk, Pfizer, Bayer, Anji, Boehringer Ingelheim, Valo, Lilly, and Vertex. KC reports grant funding from Boehringer Ingelheim, and support to attend meetings from Boehringer Ingelheim via the University of Oxford. LSH reports unpaid leadership roles as Editor of the Malaysian Dialysis Transplant Registry, and Chairman of the Nephrology Medical Education Committee of the Malaysian Medical Council. TK reports grant funding from Nippon Boehringer Ingelheim Co, Eli Lilly Japan, Kyowa Kirin Co, MSD Corporation, Daiichi Sankyo Co, Novo Nordisk, Sanofi, Takeda Pharmaceutical Co, Astellas Pharma, Ono Pharmaceutical Co, Mitsubishi Tanabe Pharma Corporation, and Taisho Pharmaceutical Co; and honoraria from MSD Corporation, Takeda Pharmaceutical Co, Mitsubishi Tanabe Pharma Corporation, Astellas Pharma, Teijin Pharma, Ono Pharmaceutical Co, AstraZeneca, Sumitomo Pharma Co, Sanofi, Eli Lilly Japan, Nippon Boehringer Ingelheim Co, Novo Nordisk Pharma, Daiichi Sankyo Co, FUJIFILM Toyama Chemical Co, Kowa Co, and Taisho Pharmaceutical Co. MN reports grant funding from Boehringer Ingelheim, Kyowa-Kirin, Daiichi-Sankyo, Astellas, Tanabe-Mitsubishi, JT, Chuga, Torii, Takeda, and Bayer; consulting fees from Kyowa-Kirin, Astellas, Daiichi-Sankyo, Tanabe-Mitsubishi, JT, and Boehringer Ingelheim; and honoraria from Kyowa-Kirin, Astellas, AstraZeneca, GSK, Daiichi-Sankyo, Tanabe-Mitsubishi, Chugai, and Boehringer Ingelheim. AL reports grant funding from Boehringer Ingelheim; honoraria from Boehringer Ingelheim, AstraZeneca, and GSK; and support to attend meetings or travel from Novo Nordisk. DZIC reports grant funding from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck & Co, Sanofi, CSL-Behring, and Novo Nordisk; and consulting fees or honoraria from Boehringer Ingelheim-Lilly, Merck, AstraZeneca, Sanofi, Mitsubishi-Tanabe, AbbVie, Janssen, Bayer, Prometic, Bristol Myers Squibb, Maze, Gilead, CSL-Behring, Otsuka, Novartis, Youngene, Lexicon, Inversago, GSK, and Novo Nordisk; support for attending meetings or travel from Boehringer Ingelheim-Lilly, Merck, AstraZeneca, Sanofi, Janssen, Bayer, and Novo Nordisk; and receipt of equipment, materials, drugs, medical writing, gifts, or other services from AstraZeneca. APM reports leadership or fiduciary roles in other boards, societies, committees, or advocacy groups for Sanofi, AstraZeneca, and Novartis, outside the present work. SG reports paid leadership roles (personal payment) as an associate editor for Circulation and for the American Heart Association, and as a steering committee member for the Duke Clinical Research Institute. XR reports an unpaid consultancy role as an honorary junior consultant for Boehringer Ingelheim, and unpaid leadership roles on the European Society of Cardiology clinical practice guidelines and registries committees. KRT reports grant funding from Bayer, Travere, and Goldfinch Bio; consulting fees from Lilly, Boehringer Ingelheim, AstraZeneca, Goldfinch Bio, Novo Nordisk, Bayer, and Travere; honoraria from Lilly, AstraZeneca, Novo Nordisk, and Bayer; unpaid roles on data safety monitoring or advisory boards for the US National Institute of Diabetes and Digestive and Kidney Diseases and George Clinical Institute; and an unpaid leadership role as Chair for the Diabetic Kidney Disease Collaborative of the American Society of Nephrology. DS, MP, and SS are employees of Boehringer Ingelheim International. SB, Z-HL, JL, WL, RP, and RD declare no competing interests.
Figures


Comment in
-
Empagliflozin in chronic kidney disease: nephroprotection is independent of albuminuria, primary kidney disease, and baseline eGFR.Lancet Diabetes Endocrinol. 2024 Jan;12(1):5-8. doi: 10.1016/S2213-8587(23)00355-8. Epub 2023 Dec 4. Lancet Diabetes Endocrinol. 2024. PMID: 38061373 No abstract available.
References
-
- Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64(6):821–35. - PubMed
-
- Inker LA, Lambers Heerspink HJ, Mondal H, et al. GFR decline as an alternative end point to kidney failure in clinical trials: a meta-analysis of treatment effects from 37 randomized trials. Am J Kidney Dis. 2014;64(6):848–59. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

