HIV-1 reservoir size after neonatal antiretroviral therapy and the potential to evaluate antiretroviral-therapy-free remission (IMPAACT P1115): a phase 1/2 proof-of-concept study
- PMID: 38061376
- PMCID: PMC11094801
- DOI: 10.1016/S2352-3018(23)00236-9
HIV-1 reservoir size after neonatal antiretroviral therapy and the potential to evaluate antiretroviral-therapy-free remission (IMPAACT P1115): a phase 1/2 proof-of-concept study
Abstract
Background: Infants born with HIV-1 require lifelong antiretroviral therapy (ART). We aimed to assess whether very early ART in neonates might restrict HIV-1 reservoirs, an important step towards ART-free remission.
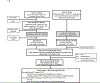
Methods: IMPAACT P1115 is an ongoing, phase 1/2, proof-of-concept study in which infants were enrolled at 30 research clinics in 11 countries (Brazil, Haiti, Kenya, Malawi, South Africa, Tanzania, Thailand, Uganda, the USA, Zambia, and Zimbabwe) into two cohorts. Infants at least 34 weeks' gestational age at high risk for in-utero HIV-1 with either untreated maternal HIV-1 (cohort 1) or who were receiving pre-emptive triple antiretroviral prophylaxis outside of the study (maternal ART permissible; cohort 2) were included. All infants initiated treatment within 48 h of life. Cohort 1 initiated three-drug nevirapine-based ART, and cohort 2 initiated three-drug nevirapine-based prophylaxis then three-drug nevirapine-based ART following HIV diagnosis by age 10 days. We added twice-daily coformulated oral ritonavir 75 mg/m2 and lopinavir 300 mg/m2 from 14 days of life and 42 weeks postmenstrual age. We discontinued nevirapine 12 weeks after two consecutive plasma HIV-1 RNA levels below limit of detection. We tracked virological suppression, safety outcomes, and meeting a predetermined biomarker profile at age 2 years (undetectable RNA since week 48, HIV-1 antibody-negative, HIV-1 DNA not detected, and normal CD4 count and CD4 percentage) to assess qualification for analytical treatment interruption. This study is registered with ClinicalTrials.gov, NCT02140255.
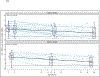
Findings: Between Jan 23, 2015, and Dec 14, 2017, 440 infants were included in cohort 1 and 20 were included in cohort 2. 54 of these infants (34 from cohort 1 and 20 from cohort 2) had confirmed in-utero HIV-1 and were enrolled to receive study ART. 33 (61%) of 54 infants were female and 21 (39%) were male. The estimated probability of maintaining undetectable plasma RNA through to 2 years was 33% (95% CI 17-49) in cohort 1 and 57% (28-78) in cohort 2. Among infants maintaining protocol-defined virological control criteria through to study week 108, seven of 11 (64%, 95% CI 31-89) in cohort 1 and five of seven (71%, 29-96) in cohort 2 had no detected HIV-1 DNA. Ten of 12 (83%, 52-100) in cohort 1 and all seven (100%, 59-100) in cohort 2 tested HIV-1 antibody-negative at week 108. Among 54 infants initiated on very early ART, ten (19%; six in cohort 1 and four in cohort 2) met all criteria for possible analytical treatment interruption. Reversible grade 3 or 4 adverse events occurred in 15 (44%) of 34 infants in cohort 1 and seven (35%) of 20 infants in cohort 2.
Interpretation: Very early ART for in-utero HIV-1 can achieve sustained virological suppression in association with biomarkers indicating restricted HIV-1 reservoirs by age 2 years, which might enable potential ART-free remission.
Funding: National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health.
Copyright © 2024 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MM receives research support from Gilead Sciences, ViiV Healthcare, and Merck. EVC serves as a consultant to Melinta Pharmaceuticals. EGC's spouse holds an equity interest in AbbVie. All other authors declare no competing interests.
Figures




Comment in
-
Super early treatment for HIV acquired in utero.Lancet HIV. 2024 Jan;11(1):e3-e4. doi: 10.1016/S2352-3018(23)00260-6. Epub 2023 Dec 4. Lancet HIV. 2024. PMID: 38061375 No abstract available.
References
-
- WHO. Mother-to-child transmission of HIV. https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/p... (accessed 3/14/2023 2023).
-
- Deeks SG, Archin N, Cannon P, et al. Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021. Nat Med 2021; 27(12): 2085–98. - PubMed
-
- Butler KM, Gavin P, Coughlan S, et al. Rapid viral rebound after 4 years of suppressive therapy in a seronegative HIV-1 infected infant treated from birth. Pediatr Infect Dis J 2015; 34(3): e48–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 75N91019D00024/CA/NCI NIH HHS/United States
- P30 AI094189/AI/NIAID NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- R01 AI150412/AI/NIAID NIH HHS/United States
- UM1 AI069530/AI/NIAID NIH HHS/United States
- U01 AI068616/AI/NIAID NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- UM1 AI164566/AI/NIAID NIH HHS/United States
- UM1 AI069436/AI/NIAID NIH HHS/United States
- UM1 AI069536/AI/NIAID NIH HHS/United States
- UM1 AI106716/AI/NIAID NIH HHS/United States
- U01 AI069436/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

