New insights on mitochondrial heteroplasmy observed in ovarian diseases
- PMID: 38061426
- PMCID: PMC11519015
- DOI: 10.1016/j.jare.2023.11.033
New insights on mitochondrial heteroplasmy observed in ovarian diseases
Abstract
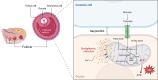
Background: The reportedly high mutation rate of mitochondrial DNA (mtDNA) may be attributed to the absence of histone protection and complete repair mechanisms. Mitochondrial heteroplasmy refers to the coexistence of wild-type and mutant mtDNA. Most healthy individuals carry a low point mutation load (<1 %) in their mtDNA, typically without any discernible phenotypic effects. However, as it exceeds a certain threshold, it may cause the onset of various diseases. Since the ovary is a highly energy-intensive organ, it relies heavily on mitochondrial function. Mitochondrial heteroplasmy can potentially contribute to a variety of significant ovarian disorders.
Aim of review: In this review, we have elucidated the close relationship between mtDNA heteroplasmy and ovarian diseases, and summarized novel avenues and strategies for the potential treatment of these ovarian diseases.
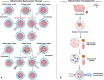
Key scientific concepts of review: Mitochondrial heteroplasmy can potentially contribute to a variety of significant ovarian disorders, including polycystic ovary syndrome, premature ovarian insufficiency, and endometriosis. Current strategies related to mitochondrial heteroplasmy are untargeted and have low bioavailability. Nanoparticle delivery systems loaded with mitochondrial modulators, mitochondrial replacement/transplantation therapy, and mitochondria-targeted gene editing therapy may offer promising paths towards potentially more effective treatments for these diseases, despite ongoing challenges.
Keywords: Endometriosis; Mitochondria-targeted therapy; Mitochondrial heteroplasmy; Polycystic ovary syndrome; Premature ovarian insufficiency.
Copyright © 2024. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

