Lycium barbarum polysaccharide alleviates DSS-induced chronic ulcerative colitis by restoring intestinal barrier function and modulating gut microbiota
- PMID: 38061697
- PMCID: PMC10836275
- DOI: 10.1080/07853890.2023.2290213
Lycium barbarum polysaccharide alleviates DSS-induced chronic ulcerative colitis by restoring intestinal barrier function and modulating gut microbiota
Abstract
Purpose: This study examined the protective effects and mechanism of Lycium barbarum polysaccharides (LBP) in the context of intestinal barrier function and intestinal microbiota in mice with dextran sulfate sodium (DSS)-induced chronic ulcerative colitis (UC).
Methods: C57BL/6J male mice were assigned to a standard normal diet without DSS (control group), a normal diet with DSS (DSS group, 2% DSS given discontinuously for 3 weeks) or a normal diet supplemented with LBP (1% dry feed weight, LBP group, 2% DSS given discontinuously for 3 weeks) for a total of 8 weeks, at which point colonic tissues and caecal contents were collected.
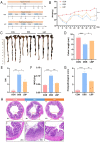
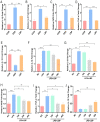
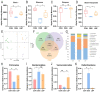
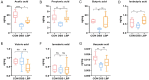
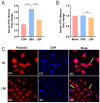
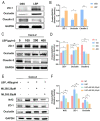
Results: LBP exerted a significant effect against colitis by increasing body weight, colon length, DAI and histopathological scores. LBP inhibited proinflammatory cytokines (IL-1β, IL-6, iNOS and TNF-α) expression, improved anti-inflammatory cytokine (IL-10) expression, promoted the expression of tight junction proteins (Occludin and ZO-1) via nuclear factor erythroid 2-related factor 2 (Nrf2) activation and decreased Claudin-2 expression to maintain the intestinal mucosal barrier. In addition, the abundances of some probiotics (Ruminococcaceae, Lactobacillus, Butyricicoccus, and Akkermansia) were decreased with DSS treatment but increased obviously with LBP treatment. And LBP reduced the abundance of conditional pathogens associated with UC (Mucispirillum and Sutterella). Furthermore, LBP improved the production of short-chain fatty acids (SCFAs), including acetic acid, propionic acid, butyric acid and isobutyric acid.
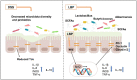
Conclusion: LBP can alleviate DSS-induced UC by regulating inflammatory cytokines and tight junction proteins. Moreover, LBP promotes probiotics, suppresses conditional pathogens and increases SCFAs production, showing a strong prebiotic effect.
Keywords: Lycium barbarum polysaccharides; gut microbiota; tight junction proteins; ulcerative colitis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
