The amygdala is not necessary for the familiarity aspect of recognition memory
- PMID: 38062014
- PMCID: PMC10703781
- DOI: 10.1038/s41467-023-43906-8
The amygdala is not necessary for the familiarity aspect of recognition memory
Abstract
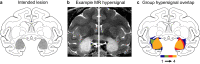
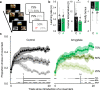
Dual-process accounts of item recognition posit two memory processes: slow but detailed recollection, and quick but vague familiarity. It has been proposed, based on prior rodent work, that the amygdala is critical for the familiarity aspect of item recognition. Here, we evaluated this proposal in male rhesus monkeys (Macaca mulatta) with selective bilateral excitotoxic amygdala damage. We used four established visual memory tests designed to assess different aspects of familiarity, all administered on touchscreen computers. Specifically, we assessed monkeys' tendencies to make low-latency false alarms, to make false alarms to recently seen lures, to produce curvilinear ROC curves, and to discriminate stimuli based on repetition across days. Three of the four tests showed no familiarity impairment and the fourth was explained by a deficit in reward processing. Consistent with this, amygdala damage did produce an anticipated deficit in reward processing in a three-arm-bandit gambling task, verifying the effectiveness of the lesions. Together, these results contradict prior rodent work and suggest that the amygdala is not critical for the familiarity aspect of item recognition.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Recognition errors suggest fast familiarity and slow recollection in rhesus monkeys.Learn Mem. 2013 Jul 17;20(8):431-7. doi: 10.1101/lm.029223.112. Learn Mem. 2013. PMID: 23864646 Free PMC article.
-
Double dissociation of selective recollection and familiarity impairments following two different surgical treatments for temporal-lobe epilepsy.Neuropsychologia. 2010 Jul;48(9):2640-7. doi: 10.1016/j.neuropsychologia.2010.05.010. Epub 2010 May 11. Neuropsychologia. 2010. PMID: 20466009
-
Amygdala lesions selectively impair familiarity in recognition memory.Nat Neurosci. 2011 Sep 25;14(11):1416-7. doi: 10.1038/nn.2919. Nat Neurosci. 2011. PMID: 21946327 Free PMC article.
-
An animal model of amnesia that uses Receiver Operating Characteristics (ROC) analysis to distinguish recollection from familiarity deficits in recognition memory.Neuropsychologia. 2010 Jul;48(8):2281-9. doi: 10.1016/j.neuropsychologia.2009.09.015. Epub 2009 Sep 20. Neuropsychologia. 2010. PMID: 19772865 Free PMC article.
-
ROC in animals: uncovering the neural substrates of recollection and familiarity in episodic recognition memory.Conscious Cogn. 2010 Sep;19(3):816-28. doi: 10.1016/j.concog.2010.06.023. Epub 2010 Aug 5. Conscious Cogn. 2010. PMID: 20691613 Free PMC article. Review.
Cited by
-
Unpacking the Medial Temporal Lobe: Separating Recollection and Familiarity.Hippocampus. 2025 Sep;35(5):e70033. doi: 10.1002/hipo.70033. Hippocampus. 2025. PMID: 40919742 Free PMC article.
-
Beyond faces: the contribution of the amygdala to visual processing in the macaque brain.Cereb Cortex. 2024 Jun 4;34(6):bhae245. doi: 10.1093/cercor/bhae245. Cereb Cortex. 2024. PMID: 38864574 Free PMC article.
-
Prefrontal-Amygdala Pathways for Object and Social Value Representation.J Cogn Neurosci. 2024 Dec 1;36(12):2687-2696. doi: 10.1162/jocn_a_02144. J Cogn Neurosci. 2024. PMID: 38527093 Free PMC article.
-
Significant variations in tolerance to clothianidin and pirimiphos-methyl in Anopheles gambiae and Anopheles funestus populations during a dramatic malaria resurgence despite sustained indoor residual spraying in Uganda.Parasit Vectors. 2025 Jun 23;18(1):237. doi: 10.1186/s13071-025-06867-z. Parasit Vectors. 2025. PMID: 40551263 Free PMC article.
References
-
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J. Mem. Lang. 2002;46:441–517. doi: 10.1006/jmla.2002.2864. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

