Cortico-muscular connectivity is modulated by passive and active Lokomat-assisted Gait
- PMID: 38062035
- PMCID: PMC10703891
- DOI: 10.1038/s41598-023-48072-x
Cortico-muscular connectivity is modulated by passive and active Lokomat-assisted Gait
Abstract
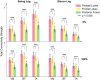
The effects of robotic-assisted gait (RAG) training, besides conventional therapy, on neuroplasticity mechanisms and cortical integration in locomotion are still uncertain. To advance our knowledge on the matter, we determined the involvement of motor cortical areas in the control of muscle activity in healthy subjects, during RAG with Lokomat, both with maximal guidance force (100 GF-passive RAG) and without guidance force (0 GF-active RAG) as customary in rehabilitation treatments. We applied a novel cortico-muscular connectivity estimation procedure, based on Partial Directed Coherence, to jointly study source localized EEG and EMG activity during rest (standing) and active/passive RAG. We found greater cortico-cortical connectivity, with higher path length and tendency toward segregation during rest than in both RAG conditions, for all frequency bands except for delta. We also found higher cortico-muscular connectivity in distal muscles during swing (0 GF), and stance (100 GF), highlighting the importance of direct supraspinal control to maintain balance, even when gait is supported by a robotic exoskeleton. Source-localized connectivity shows that this control is driven mainly by the parietal and frontal lobes. The involvement of many cortical areas also in passive RAG (100 GF) justifies the use of the 100 GF RAG training for neurorehabilitation, with the aim of enhancing cortical-muscle connections and driving neural plasticity in neurological patients.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Calabrò RS, et al. What does evidence tell us about the use of gait robotic devices in patients with multiple sclerosis? A comprehensive systematic review on functional outcomes and clinical recommendations. Eur. J. Phys. Rehabil. Med. 2021;7:841–849. - PubMed
-
- Mehrholz J, Hädrich A, Platz T, Kugler J, Pohl M. Electromechanical and robot-assisted arm training for improving generic activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst. Rev. 2012;6:CD006876. - PubMed
-
- Kleim, J. A. & Jones, T. A. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage (2008). - PubMed
MeSH terms
Grants and funding
- PRIN-2017 'INSPECT'/Ministero dell'Istruzione, dell'Università e della Ricerca
- PRIN-2017 'INSPECT'/Ministero dell'Istruzione, dell'Università e della Ricerca
- FAS Salute 2018/Regione Toscana (Italy)
- A multiscale integrated approach to the study of the nervous system in health and disease - MNESYS/Ministry of University and Research and European Union
LinkOut - more resources
Full Text Sources

