Cancer CD39 drives metabolic adaption and mal-differentiation of CD4+ T cells in patients with non-small-cell lung cancer
- PMID: 38062068
- PMCID: PMC10703826
- DOI: 10.1038/s41419-023-06336-4
Cancer CD39 drives metabolic adaption and mal-differentiation of CD4+ T cells in patients with non-small-cell lung cancer
Abstract
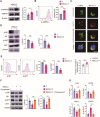
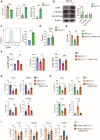
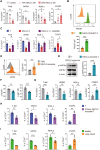
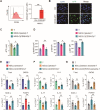
While ectonucleotidase CD39 is a cancer therapeutic target in clinical trials, its direct effect on T-cell differentiation in human non-small-cell lung cancer (NSCLC) remains unclear. Herein, we demonstrate that human NSCLC cells, including tumor cell lines and primary tumor cells from clinical patients, efficiently drive the metabolic adaption of human CD4+ T cells, instructing differentiation of regulatory T cells while inhibiting effector T cells. Of importance, NSCLC-induced T-cell mal-differentiation primarily depends on cancer CD39, as this can be fundamentally blocked by genetic depletion of CD39 in NSCLC. Mechanistically, NSCLC cells package CD39 into their exosomes and transfer such CD39-containing exosomes into interacting T cells, resulting in ATP insufficiency and AMPK hyperactivation. Such CD39-dependent NSCLC-T cell interaction holds well in patients-derived primary tumor cells and patient-derived organoids (PDOs). Accordingly, genetic depletion of CD39 alone or in combination with the anti-PD-1 immunotherapy efficiently rescues effector T cell differentiation, instigates anti-tumor T cell immunity, and inhibits tumor growth of PDOs. Together, targeting cancer CD39 can correct the mal-differentiation of CD4+ T cells in human NSCLC, providing in-depth insight into therapeutic CD39 inhibitors.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
[Expression levels of co-inhibitory molecules CTLA-4, LAG-3, PD-1 and CD39 on CD4⁺ T cells correlate with progression of non-small cell lung cancer].Zhonghua Zhong Liu Za Zhi. 2014 Jun;36(6):424-9. Zhonghua Zhong Liu Za Zhi. 2014. PMID: 25241783 Chinese.
-
Ectonucleotidase CD73 and CD39 expression in non-small cell lung cancer relates to hypoxia and immunosuppressive pathways.Life Sci. 2020 Oct 15;259:118389. doi: 10.1016/j.lfs.2020.118389. Epub 2020 Sep 6. Life Sci. 2020. PMID: 32898522
-
Antisense oligonucleotide targeting CD39 improves anti-tumor T cell immunity.J Immunother Cancer. 2019 Mar 12;7(1):67. doi: 10.1186/s40425-019-0545-9. J Immunother Cancer. 2019. PMID: 30871609 Free PMC article.
-
ENTPD1/CD39 is a promising therapeutic target in oncology.Oncogene. 2013 Apr 4;32(14):1743-51. doi: 10.1038/onc.2012.269. Epub 2012 Jul 2. Oncogene. 2013. PMID: 22751118 Review.
-
CD39 Regulation and Functions in T Cells.Int J Mol Sci. 2021 Jul 28;22(15):8068. doi: 10.3390/ijms22158068. Int J Mol Sci. 2021. PMID: 34360833 Free PMC article. Review.
Cited by
-
Non-small cell lung cancer organoids: Advances and challenges in current applications.Chin J Cancer Res. 2024 Oct 30;36(5):455-473. doi: 10.21147/j.issn.1000-9604.2024.05.01. Chin J Cancer Res. 2024. PMID: 39539817 Free PMC article.
-
Unraveling the Connection: Extracellular Vesicles and Non-Small Cell Lung Cancer.Int J Nanomedicine. 2024 Aug 9;19:8139-8157. doi: 10.2147/IJN.S477851. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39139506 Free PMC article. Review.
-
Identification of prognostic biomarker of non-small cell lung cancer based on mitochondrial permeability transition-driven necrosis-related genes and determination of anti-tumor effect of ARL14.Hereditas. 2025 Feb 3;162(1):16. doi: 10.1186/s41065-025-00379-7. Hereditas. 2025. PMID: 39901294 Free PMC article.
-
Tumor-infiltrating lymphocytes in NSCLC: from immune surveillance to immunotherapy.Front Immunol. 2025 Jul 25;16:1610998. doi: 10.3389/fimmu.2025.1610998. eCollection 2025. Front Immunol. 2025. PMID: 40787464 Free PMC article. Review.
-
Electrochemical detection of miRNA using commercial and hand-made screen-printed electrodes: liquid biopsy for cancer management as case of study.ChemistryOpen. 2024 Jul;13(7):e202300203. doi: 10.1002/open.202300203. Epub 2024 Feb 9. ChemistryOpen. 2024. PMID: 38333968 Free PMC article.
References
-
- J. F, M. E, F. L, M. C, L. M, Piñeros M ea. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer; 2020. 2021 [cited 2021 August] Available from: https://gco.iarc.fr/today
-
- Schoenfeld JD, Giobbie-Hurder A, Ranasinghe S, Kao KZ, Lako A, Tsuji J, et al. Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2022;23:279–91. doi: 10.1016/S1470-2045(21)00658-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

