Platelet-activating factor and protease-activated receptor 2 cooperate to promote neutrophil recruitment and lung inflammation through nuclear factor-kappa B transactivation
- PMID: 38062077
- PMCID: PMC10703791
- DOI: 10.1038/s41598-023-48365-1
Platelet-activating factor and protease-activated receptor 2 cooperate to promote neutrophil recruitment and lung inflammation through nuclear factor-kappa B transactivation
Abstract
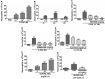
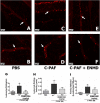
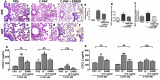
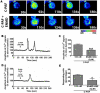
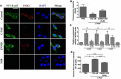
Although it is well established that platelet-activated receptor (PAF) and protease-activated receptor 2 (PAR2) play a pivotal role in the pathophysiology of lung and airway inflammatory diseases, a role for a PAR2-PAFR cooperation in lung inflammation has not been investigated. Here, we investigated the role of PAR2 in PAF-induced lung inflammation and neutrophil recruitment in lungs of BALB/c mice. Mice were pretreated with the PAR2 antagonist ENMD1068, PAF receptor (PAFR) antagonist WEB2086, or aprotinin prior to intranasal instillation of carbamyl-PAF (C-PAF) or the PAR2 agonist peptide SLIGRL-NH2 (PAR2-AP). Leukocyte infiltration in bronchoalveolar lavage fluid (BALF), C-X-C motif ligand 1 (CXCL)1 and CXCL2 chemokines, myeloperoxidase (MPO), and N-acetyl-glycosaminidase (NAG) levels in BALF, or lung inflammation were evaluated. Intracellular calcium signaling, PAFR/PAR2 physical interaction, and the expression of PAR2 and nuclear factor-kappa B (NF-КB, p65) transcription factor were investigated in RAW 264.7 cells stimulated with C-PAF in the presence or absence of ENMD1068. C-PAF- or PAR2-AP-induced neutrophil recruitment into lungs was inhibited in mice pretreated with ENMD1068 and aprotinin or WEB2086, respectively. PAR2 blockade impaired C-PAF-induced neutrophil rolling and adhesion, lung inflammation, and production of MPO, NAG, CXCL1, and CXCL2 production in lungs of mice. PAFR activation reduced PAR2 expression and physical interaction of PAR2 and PAFR; co-activation is required for PAFR/PAR2 physical interaction. PAR2 blockade impaired C-PAF-induced calcium signal and NF-κB p65 translocation in RAW 264.7 murine macrophages. This study provides the first evidence for a cooperation between PAFR and PAR2 mediating neutrophil recruitment, lung inflammation, and macrophage activation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

