Colchicine Attenuates Microvascular Obstruction after Myocardial Ischemia-Reperfusion Injury by Inhibiting the Proliferation of Neutrophil in Bone Marrow
- PMID: 38062310
- PMCID: PMC11954697
- DOI: 10.1007/s10557-023-07528-y
Colchicine Attenuates Microvascular Obstruction after Myocardial Ischemia-Reperfusion Injury by Inhibiting the Proliferation of Neutrophil in Bone Marrow
Abstract
Purpose: Complete and rapid recanalization of blood flow by percutaneous coronary intervention (PCI) is the most effective intervention for patients with ST-segment elevation myocardial infarction (STEMI). However, myocardial ischemia/reperfusion (I/R) injury leads to microvascular obstruction (MVO), limiting its efficacy. Colchicine can reduce myocardial I/R injury, but its effect on MVO is unclear. Hence, this study aimed to assess the role and mechanism of colchicine on MVO.
Methods: Clinical data on STEMI patients with PCI were collected and risk factors related to MVO were analyzed. The rat myocardial I/R model was established to evaluate the MVO by thioflavin S staining. The myocardial I/R model of mice was treated with PBS or colchicine at the reperfusion. The effect of colchicine on cardiomyocyte apoptosis after I/R was evaluated by TUNEL and expression of cleaved caspase-3. ROS levels were detected in H9c2 cells to evaluate the colchicine effect on myocardial oxidative stress. Moreover, the mechanism through which colchicine attenuated MVO was examined using flow cytometry, WB, ELISA, immunohistochemistry, bioinformatics analysis, and immunofluorescence.
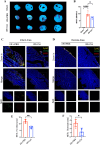
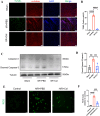
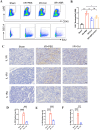
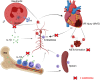
Results: Multivariate analysis showed that elevated neutrophils were associated with extensive MVO. Colchicine could attenuate MVO and reduce neutrophil recruitment and NETs formation after myocardial I/R. In addition, colchicine inhibited cardiomyocyte apoptosis in vivo and ROS levels in vitro. Furthermore, colchicine inhibited neutrophil proliferation in the bone marrow (BM) by inhibiting the S100A8/A9 inflammatory signaling pathway.
Conclusions: Colchicine attenuated MVO after myocardial I/R injury by inhibiting the proliferation of neutrophils in BM through the neutrophil-derived S100A8/A9 inflammatory signaling pathway.
Keywords: Colchicine; Microvascular obstruction; Myocardial ischemia/reperfusion injury; NETs; S100A8/A9.
© 2023. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval and Consent to Participate: The current study was approved by the Ethics Committee of Nanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University. All animal experiments were approved by the Institutional Ethics Committee of Nanjing Drum Hospital. Consent for Publication: Not applicable. Competing Interests: The authors declare that they have no competing interests.
Figures


Similar articles
-
Excessive accumulation of epicardial adipose tissue promotes microvascular obstruction formation after myocardial ischemia/reperfusion through modulating macrophages polarization.Cardiovasc Diabetol. 2024 Jul 5;23(1):236. doi: 10.1186/s12933-024-02342-8. Cardiovasc Diabetol. 2024. PMID: 38970123 Free PMC article.
-
S100a8/a9 Signaling Causes Mitochondrial Dysfunction and Cardiomyocyte Death in Response to Ischemic/Reperfusion Injury.Circulation. 2019 Aug 27;140(9):751-764. doi: 10.1161/CIRCULATIONAHA.118.039262. Epub 2019 Jun 21. Circulation. 2019. PMID: 31220942
-
MSC-Derived Exosomes Mitigate Myocardial Ischemia/Reperfusion Injury by Reducing Neutrophil Infiltration and the Formation of Neutrophil Extracellular Traps.Int J Nanomedicine. 2024 Mar 5;19:2071-2090. doi: 10.2147/IJN.S436925. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38476275 Free PMC article.
-
Mechanistic Role of mPTP in Ischemia-Reperfusion Injury.Adv Exp Med Biol. 2017;982:169-189. doi: 10.1007/978-3-319-55330-6_9. Adv Exp Med Biol. 2017. PMID: 28551787 Review.
-
Morphine and myocardial ischaemia-reperfusion.Eur J Pharmacol. 2021 Jan 15;891:173683. doi: 10.1016/j.ejphar.2020.173683. Epub 2020 Oct 26. Eur J Pharmacol. 2021. PMID: 33121952 Review.
Cited by
-
Immune in myocardial ischemia/reperfusion injury: potential mechanisms and therapeutic strategies.Front Immunol. 2025 May 8;16:1558484. doi: 10.3389/fimmu.2025.1558484. eCollection 2025. Front Immunol. 2025. PMID: 40406107 Free PMC article. Review.
-
Immuno-inflammatory pathogenesis in ischemic heart disease: perception and knowledge for neutrophil recruitment.Front Immunol. 2024 Jul 10;15:1411301. doi: 10.3389/fimmu.2024.1411301. eCollection 2024. Front Immunol. 2024. PMID: 39050842 Free PMC article. Review.
-
NETosis in myocardial ischemia-reperfusion injury: From mechanisms to therapies (Review).Biomed Rep. 2025 May 13;23(1):113. doi: 10.3892/br.2025.1991. eCollection 2025 Jul. Biomed Rep. 2025. PMID: 40420974 Free PMC article. Review.
-
Can We Leave No Metal Behind? DCB vs. DES in Large Coronary Arteries.Cardiovasc Drugs Ther. 2024 Dec;38(6):1077-1078. doi: 10.1007/s10557-024-07623-8. Epub 2024 Aug 23. Cardiovasc Drugs Ther. 2024. PMID: 39177719 No abstract available.
-
Unraveling the Mechanisms of S100A8/A9 in Myocardial Injury and Dysfunction.Curr Issues Mol Biol. 2024 Sep 2;46(9):9707-9720. doi: 10.3390/cimb46090577. Curr Issues Mol Biol. 2024. PMID: 39329929 Free PMC article. Review.
References
-
- Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. 2017;376(21):2053–64. 10.1056/NEJMra1606915. - PubMed
-
- Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. 2022;327(7):662–75. 10.1001/jama.2022.0358. - PubMed
-
- Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 2016;37(13):1024–33. 10.1093/eurheartj/ehv484. - PubMed
-
- Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008;117(24):3152–6. 10.1161/CIRCULATIONAHA.107.742312. - PubMed
-
- Symons R, Pontone G, Schwitter J, et al. Long-term incremental prognostic value of cardiovascular magnetic resonance after st-segment elevation myocardial infarction: a study of the collaborative registry on CMR in STEMI. JACC Cardiovasc Imaging. 2018;11(6):813–25. 10.1016/j.jcmg.2017.05.023. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

