The hypothalamic steroidogenic pathway mediates susceptibility to inflammation-evoked depression in female mice
- PMID: 38062440
- PMCID: PMC10704691
- DOI: 10.1186/s12974-023-02976-7
The hypothalamic steroidogenic pathway mediates susceptibility to inflammation-evoked depression in female mice
Abstract
Background: Depression is two-to-three times more frequent among women. The hypothalamus, a sexually dimorphic area, has been implicated in the pathophysiology of depression. Neuroinflammation-induced hypothalamic dysfunction underlies behaviors associated with depression. The lipopolysaccharide (LPS)-induced mouse model of depression has been well-validated in numerous laboratories, including our own, and is widely used to investigate the relationship between neuroinflammation and depression. However, the sex-specific differences in metabolic alterations underlying depression-associated hypothalamic neuroinflammation remain unknown.
Methods: Here, we employed the LPS-induced mouse model of depression to investigate hypothalamic metabolic changes in both male and female mice using a metabolomics approach. Through bioinformatics analysis, we confirmed the molecular pathways and biological processes associated with the identified metabolites. Furthermore, we employed quantitative real-time PCR, enzyme-linked immunosorbent assay, western blotting, and pharmacological interventions to further elucidate the underlying mechanisms.
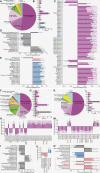
Results: A total of 124 and 61 differential metabolites (DMs) were detected in male and female mice with depressive-like behavior, respectively, compared to their respective sex-matched control groups. Moreover, a comparison between female and male model mice identified 37 DMs. We capitalized on biochemical clustering and functional enrichment analyses to define the major metabolic changes in these DMs. More than 55% of the DMs clustered into lipids and lipid-like molecules, and an imbalance in lipids metabolism was presented in the hypothalamus. Furthermore, steroidogenic pathway was confirmed as a potential sex-specific pathway in the hypothalamus of female mice with depression. Pregnenolone, an upstream component of the steroid hormone biosynthesis pathway, was downregulated in female mice with depressive-like phenotypes but not in males and had considerable relevance to depressive-like behaviors in females. Moreover, exogenous pregnenolone infusion reversed depressive-like behaviors in female mice with depression. The 5α-reductase type I (SRD5A1), a steroidogenic hub enzyme involved in pregnenolone metabolism, was increased in the hypothalamus of female mice with depression. Its inhibition increased hypothalamic pregnenolone levels and ameliorated depressive-like behaviors in female mice with depression.
Conclusions: Our study findings demonstrate a marked sexual dimorphism at the metabolic level in depression, particularly in hypothalamic steroidogenic metabolism, identifying a potential sex-specific pathway in female mice with depressive-like behaviors.
Keywords: Depression; Hypothalamus; Neuroinflammation; Neurosteroid biosynthesis; Pregnenolone; Sex.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
- 22JR11RA246/the Gansu Provincial Natural Science Foundation of China
- 21JR7RA650/the Gansu Provincial Natural Science Foundation of China
- 21JR7RA630/the Gansu Provincial Natural Science Foundation of China
- 21JR1RA004/the Gansu Provincial Natural Science Foundation of China
- 82160534/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical

