GCLiPP: global crosslinking and protein purification method for constructing high-resolution occupancy maps for RNA binding proteins
- PMID: 38062486
- PMCID: PMC10701951
- DOI: 10.1186/s13059-023-03125-2
GCLiPP: global crosslinking and protein purification method for constructing high-resolution occupancy maps for RNA binding proteins
Abstract
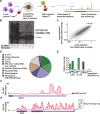
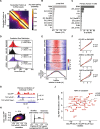
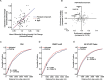
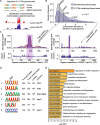
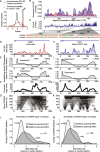
GCLiPP is a global RNA interactome capture method that detects RNA-binding protein (RBP) occupancy transcriptome-wide. GCLiPP maps RBP-occupied sites at a higher resolution than phase separation-based techniques. GCLiPP sequence tags correspond with known RBP binding sites and are enriched for sites detected by RBP-specific crosslinking immunoprecipitation (CLIP) for abundant cytosolic RBPs. Comparison of human Jurkat T cells and mouse primary T cells uncovers shared peaks of GCLiPP signal across homologous regions of human and mouse 3' UTRs, including a conserved mRNA-destabilizing cis-regulatory element. GCLiPP signal overlapping with immune-related SNPs uncovers stabilizing cis-regulatory regions in CD5, STAT6, and IKZF1.
Keywords: Cis-regulatory elements; Post-transcriptional regulation; RNA-binding proteins (RBP); T cells.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

