Subcutaneous batoclimab in generalized myasthenia gravis: Results from a Phase 2a trial with an open-label extension
- PMID: 38062618
- PMCID: PMC10791011
- DOI: 10.1002/acn3.51946
Subcutaneous batoclimab in generalized myasthenia gravis: Results from a Phase 2a trial with an open-label extension
Abstract
Objectives: To assess the safety, tolerability, and key pharmacodynamic effects of subcutaneous batoclimab, a fully human anti-neonatal Fc receptor monoclonal antibody, in patients with generalized myasthenia gravis and anti-acetylcholine receptor antibodies.
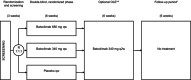
Methods: A Phase 2a, proof-of-concept, randomized, double-blind, placebo-controlled trial is described. Eligible patients were randomized (1:1:1) to receive once-weekly subcutaneous injections of batoclimab 340 mg, batoclimab 680 mg, or matching placebo for 6 weeks. Subsequently, all patients could enter an open-label extension study where they received batoclimab 340 mg once every 2 weeks for 6 weeks. Primary endpoints were safety, tolerability, and change from baseline in total immunoglobulin G, immunoglobulin G subclasses, and anti-acetylcholine receptor antibodies at 6 weeks post-baseline. Secondary endpoints included changes from baseline to 6 weeks post-baseline for Myasthenia Gravis Activities of Daily Living, Quantitative Myasthenia Gravis, Myasthenia Gravis Composite, and revised 15-item Myasthenia Gravis Quality of Life scores.
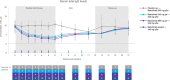
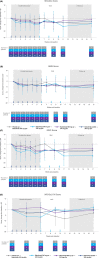
Results: Seventeen patients were randomized to batoclimab 680 mg (n = 6), batoclimab 340 mg (n = 5), or placebo (n = 6). Batoclimab was associated with significantly greater reductions in total immunoglobulin G and anti-acetylcholine receptor antibodies from baseline to 6 weeks post-baseline than placebo. Reductions in immunoglobulin G subclasses were generally consistent with total immunoglobulin G. While clinical measures showed directionally favorable improvements over time, the study was not powered to draw conclusions about therapeutic efficacy. No safety issues were identified.
Interpretation: The safety profile, pharmacodynamics, and preliminary clinical benefits observed in this study support further investigation of subcutaneous batoclimab injections as a potential patient-administered therapy for seropositive generalized myasthenia gravis.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Richard J. Nowak reports research support from the National Institutes of Health, Genentech, Inc., Alexion Pharmaceuticals, Inc., argenx, Annexon Biosciences, Inc., Ra Pharmaceuticals, Inc. (now UCB S.A.), the Myasthenia Gravis Foundation of America, Inc., Momenta Pharmaceuticals, Inc., Immunovant, Inc., Grifols, S.A., and Viela Bio, Inc. (Horizon Therapeutics plc). Dr. Nowak has also served as a consultant and advisor for Alexion Pharmaceuticals, Inc., argenx, Cabaletta Bio, Inc., CSL Behring, Grifols, S.A., Ra Pharmaceuticals, Inc. (now UCB S.A.), Immunovant, Inc., Momenta Pharmaceuticals, Inc., and Viela Bio, Inc. (Horizon Therapeutics plc). Ari Breiner reports grant support from the Muscular Dystrophy Association and Muscular Dystrophy Canada. Dr. Breiner has served as an advisor for Alnylam, and has received speaker fees from Mitsubishi‐Tanabe. Vera Bril has served as a consultant for Grifols, CSL, UCB, argenx, Takeda, Alnylam Octapharma, Pfizer, Powell Mansfield Inc, Akcea, Ionis Immunovant, Sanofi, Momenta (J&J), Roche, Janssen, AZ‐Alexion, and NovoNordisk. Dr. Bril also reports research support from AZ‐Alexion, Grifols, CSL, UCB, argenx, Takeda, Octapharma, Akcea, Momenta (J&J), Immunovant, and Ionis. Jeffrey A. Allen has served as a consultant for argenx, Alexion, Annexon, CSL Behring, Takeda, ImmunoPharma, Grifols, Pfizer, Johnson and Johnson. Shaida Khan has served as a consultant for UCB Pharmaceuticals. Dr. Khan has served on advisory boards for Alexion, UCB Pharmaceuticals, argenx, and Immunovant, Inc. Dr. Khan has received research support from the Fichtenbaum Charitable Trust. Todd Levine has served as a consultant for Immunovant, Inc. Daniel H. Jacobs has no disclosures to report for the last 3 years. Gregory Sahagian received consulting and/or research fees from Biogen, UCB, argenx, Alexion and Immunovant. Zaeem A. Siddiqi has served as a consultant for Pfizer, Grifols, CSL Behring, UCB, Takeda, Alnylam. Octapharma, and AZ‐Alexion and has received research support from Pfizer AZ‐Alexion, Grifols, CSL Behring, UCB, Takeda, and Octapharma. Jing Xu: Employee of Immunovant, Inc (New York, NY). William L. Macias: Employee of Immunovant, Inc (New York, NY). Michael Benatar reports grants from the National Institutes of Health and the Muscular Dystrophy Association; as well as consulting fees for Alector, Alexion, Annexon, Arrowhead, Biogen, Cartesian, Denali, Eli Lilly, Horizon, Immunovant, Janssen, Novartis, Orphazyme, Roche, Sanofi, Takeda, UCB and UniQure. The University of Miami has licensed intellectual property to Biogen to support design of the ATLAS study.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

