Increased Progression-Free Survival with Cabozantinib Versus Placebo in Patients with Radioiodine-Refractory Differentiated Thyroid Cancer Irrespective of Prior Vascular Endothelial Growth Factor Receptor-Targeted Therapy and Tumor Histology: A Subgroup Analysis of the COSMIC-311 Study
- PMID: 38062732
- PMCID: PMC10951569
- DOI: 10.1089/thy.2023.0463
Increased Progression-Free Survival with Cabozantinib Versus Placebo in Patients with Radioiodine-Refractory Differentiated Thyroid Cancer Irrespective of Prior Vascular Endothelial Growth Factor Receptor-Targeted Therapy and Tumor Histology: A Subgroup Analysis of the COSMIC-311 Study
Abstract
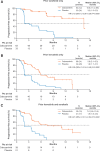
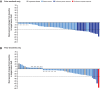
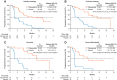
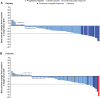
Background: Lenvatinib and sorafenib are standard of care first-line treatments for advanced, radioiodine-refractory (RAIR) differentiated thyroid cancer (DTC). However, most patients eventually become treatment-resistant and require additional therapies. The phase 3 COSMIC-311 study investigated cabozantinib in patients with RAIR DTC who progressed on lenvatinib, sorafenib, or both and showed that cabozantinib provided substantial clinical benefit. Presented in this study is an analysis of COSMIC-311 based on prior therapy and histology. Methods: Patients were randomized 2:1 (stratification: prior lenvatinib [yes/no]; age [≤65, >65 years]) to oral cabozantinib (60 mg tablet/day) or matched placebo. Eligible patients received 1-2 prior vascular endothelial growth factor receptor-targeting tyrosine kinase inhibitors for DTC (lenvatinib or sorafenib required), had a confirmed DTC diagnosis, and were refractory to or ineligible for radioiodine therapy. For this analysis, progression-free survival (PFS) and objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors version 1.1 by a blinded independent radiology committee were evaluated by prior therapy (lenvatinib only, sorafenib only, both) and histology (papillary, follicular, oncocytic, poorly differentiated). Results: Two hundred fifty-eight patients were randomized (170 cabozantinib/88 placebo) who previously received sorafenib only (n = 96), lenvatinib only (n = 102), or both (n = 60). The median follow-up was 10.1 months. The median PFS (months) with cabozantinib/placebo was 16.6/3.2 (sorafenib only: hazard ratio [HR] 0.13 [95% confidence interval, CI, 0.06-0.26]), 5.8/1.9 (lenvatinib only: HR 0.28 [95% CI 0.16-0.48]), and 7.6/1.9 (both: HR 0.27 [95% CI 0.13-0.54]). The ORR with cabozantinib/placebo was 21%/0% (sorafenib only), 4%/0% (lenvatinib only), and 8%/0% (both). Disease histology consisted of 150 papillary and 113 follicular, including 43 oncocytic and 36 poorly differentiated. The median PFS (months) with cabozantinib/placebo was 9.2/1.9 (papillary: HR 0.27 [95% CI 0.17-0.43]), 11.2/2.5 (follicular: HR 0.18 [95% CI 0.10-0.31]), 11.2/2.5 (oncocytic: HR 0.06 [95% CI 0.02-0.21]), and 7.4/1.8 (poorly differentiated: HR 0.18 [95% CI 0.08-0.43]). The ORR with cabozantinib/placebo was 15%/0% (papillary), 8%/0% (follicular), 11%/0% (oncocytic), and 9%/0% (poorly differentiated). Safety outcomes evaluated were consistent with those previously observed for the overall population. Conclusions: Results indicate that cabozantinib benefits patients with RAIR DTC, regardless of prior lenvatinib or sorafenib treatments or histology. Clinical Trial Registration Number: NCT03690388.
Keywords: COSMIC-311; cabozantinib; follicular; lenvatinib; papillary; radioiodine-refractory differentiated thyroid cancer.
Conflict of interest statement
J.C.: Consulting or advisory role—advanced accelerator applications, Bayer, Eisai, Exelixis, Ipsen, Lilly, Merck Serono, Novartis, Pfizer, and Sanofi; Speakers' Bureau—Bayer, Eisai, Ipsen, Lilly, Merck Serono, Novartis, Pfizer, and Sanofi; Research funding—Advanced Accelerator Applications (Inst.), AstraZeneca (Inst.), Bayer (Inst.), Eisai (Inst), Ipsen (Inst.), Ipsen (Inst.), Novartis (Inst.), and Pfizer (Inst.); Travel, accommodations, expenses—Eisai, Ipsen, and Pfizer. J.K.: Consulting or advisory role—Bayer Health Care, EWO-Pharma, Exelixis, Ipsen, Eli Lilly, Loxo, and Sanofi-Genzyme; Honoraria—AstraZeneca, Bayer Health Care, Eisai, Exelixis, Ipsen, Lilly, Novartis, and Sanofi-Genzyme. J.H.: Honoraria—Novartis, Eisai, Ipsen, Angelini, Adacap, and Terumo.
B.R.: Consulting or advisory role—Eisai and Eli Lilly; Speakers' Bureau—Eisai; Honoraria—Eisai and Eli Lilly; Stock or other ownership interests—Mayne Pharma; Leadership—Mayne Pharma. S.I.S.: Consulting or advisory role—Exelixis, Lilly, and Loxo; Research funding—Exelixis; Honoraria—Eisai. B.J.: Consulting or advisory role—Sobi, AstraZeneca, Exelixis, EwoPharma, and Ipsen; Honoraria—AstraZeneca, Exelixis, Sanofi-Genzyme, Ipsen, Eisai, Bayer Health Care, and Eli Lilly; Travel, accommodations, expenses—Novartis, Ipsen, and Sanofi-Genzyme. C.-C.L.: Consulting or advisory role—AbbVie, Bayer, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Novartis, and PharmaEngine; Honoraria—Daiichi Sankyo, Eli Lilly, Novartis, and Roche; Travel, accommodations, expenses—BeiGene, Daiichi Sankyo, and Eli Lilly; Other relationship—Novartis. F.V. and E.H.: Nothing to declare.
A.O.H.: Consulting or advisory role—Eli Lilly, Knight, and Bayer; Research funding—Eli Lilly, Exelixis, Roche, and Novartis; Lecture—Bayer (CME). D.W.B.: Honoraria—Exelixis and Blueprint Medicines. D.W., R.L., and J.O.: Employees and stockholders of Exelixis, Inc. B.K.: Consulting or advisory role—ABL Bio, Cellid, Handok, CbsBioscience, Trial Informatics, and NeoImmuneTech; Research funding—Ono Pharmaceutical, MSD Oncology, and AstraZeneca; Honoraria—MSD Oncology, Merck, and AstraZeneca. M.S.B.: Consulting or advisory role—Bayer, Blueprint Medicines, Eisai, Exelixis, Lilly, and Loxo; Research funding—Bayer (Inst.), Blueprint Medicines (Inst.), Eisai (Inst.), Exelixis (Inst.), Lilly (Inst.), and Loxo (Inst.); Honoraria—Bayer, Eisai, and Lilly.
Figures

References
-
- National Cancer Institute. Cancer stat facts: Thyroid cancer. 2022. Available from: https://seer.cancer.gov/statfacts/html/thyro.html [Last Accessed: July 26, 2022].
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
