Intermittent supplementation with fisetin improves arterial function in old mice by decreasing cellular senescence
- PMID: 38062873
- PMCID: PMC10928570
- DOI: 10.1111/acel.14060
Intermittent supplementation with fisetin improves arterial function in old mice by decreasing cellular senescence
Abstract
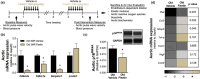
Cellular senescence and the senescence-associated secretory phenotype (SASP) contribute to age-related arterial dysfunction, in part, by promoting oxidative stress and inflammation, which reduce the bioavailability of the vasodilatory molecule nitric oxide (NO). In the present study, we assessed the efficacy of fisetin, a natural compound, as a senolytic to reduce vascular cell senescence and SASP factors and improve arterial function in old mice. We found that fisetin decreased cellular senescence in human endothelial cell culture. In old mice, vascular cell senescence and SASP-related inflammation were lower 1 week after the final dose of oral intermittent (1 week on-2 weeks off-1 weeks on dosing) fisetin supplementation. Old fisetin-supplemented mice had higher endothelial function. Leveraging old p16-3MR mice, a transgenic model allowing genetic clearance of p16INK4A -positive senescent cells, we found that ex vivo removal of senescent cells from arteries isolated from vehicle- but not fisetin-treated mice increased endothelium-dependent dilation, demonstrating that fisetin improved endothelial function through senolysis. Enhanced endothelial function with fisetin was mediated by increased NO bioavailability and reduced cellular- and mitochondrial-related oxidative stress. Arterial stiffness was lower in fisetin-treated mice. Ex vivo genetic senolysis in aorta rings from p16-3MR mice did not further reduce mechanical wall stiffness in fisetin-treated mice, demonstrating lower arterial stiffness after fisetin was due to senolysis. Lower arterial stiffness with fisetin was accompanied by favorable arterial wall remodeling. The findings from this study identify fisetin as promising therapy for clinical translation to target excess cell senescence to treat age-related arterial dysfunction.
Keywords: aging; arterial stiffness; cellular senescence; endothelial function; nutraceutical; vascular dysfunction.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
No conflicts of interest, financial, or otherwise, are declared by the authors.
Figures





References
-
- AlGhatrif, M. , Strait, J. B. , Morrell, C. H. , Canepa, M. , Wright, J. , Elango, P. , Scuteri, A. , Najjar, S. S. , Ferrucci, L. , & Lakatta, E. G. (2013). Longitudinal trajectories of arterial stiffness and the role of blood pressure: The Baltimore longitudinal study of aging. Hypertension, 62(5), 934–941. 10.1161/HYPERTENSIONAHA.113.01445 - DOI - PMC - PubMed
-
- Benjamin, E. J. , Virani, S. S. , Callaway, C. W. , Chamberlain, A. M. , Chang, A. R. , Cheng, S. , Chiuve, S. E. , Cushman, M. , Delling, F. N. , Deo, R. , de Ferranti, S. D. , Ferguson, J. F. , Fornage, M. , Gillespie, C. , Isasi, C. R. , Jiménez, M. C. , Jordan, L. C. , Judd, S. E. , Lackland, D. , … American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . (2018). Heart disease and stroke Statistics‐2018 update: A report from the American Heart Association. Circulation, 137(12), e67–e492. 10.1161/CIR.0000000000000558 - DOI - PubMed
-
- Ben‐Shlomo, Y. , Spears, M. , Boustred, C. , May, M. , Anderson, S. G. , Benjamin, E. J. , Boutouyrie, P. , Cameron, J. , Chen, C. H. , Cruickshank, J. K. , Hwang, S. J. , Lakatta, E. G. , Laurent, S. , Maldonado, J. , Mitchell, G. F. , Najjar, S. S. , Newman, A. B. , Ohishi, M. , Pannier, B. , … Wilkinson, I. B. (2014). Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta‐analysis of prospective observational data from 17,635 subjects. Journal of the American College of Cardiology, 63(7), 636–646. 10.1016/j.jacc.2013.09.063 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

