MIBlood-EV: Minimal information to enhance the quality and reproducibility of blood extracellular vesicle research
- PMID: 38063210
- PMCID: PMC10704543
- DOI: 10.1002/jev2.12385
MIBlood-EV: Minimal information to enhance the quality and reproducibility of blood extracellular vesicle research
Abstract
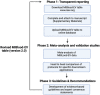
Blood is the most commonly used body fluid for extracellular vesicle (EV) research. The composition of a blood sample and its derivatives (i.e., plasma and serum) are not only donor-dependent but also influenced by collection and preparation protocols. Since there are hundreds of pre-analytical protocols and over forty variables, the development of standard operating procedures for EV research is very challenging. To improve the reproducibility of blood EV research, the International Society for Extracellular Vesicles (ISEV) Blood EV Task Force proposes standardized reporting of (i) the applied blood collection and preparation protocol and (ii) the quality of the prepared plasma and serum samples. Gathering detailed information will provide insight into the performance of the protocols and more effectively identify potential confounders in the prepared plasma and serum samples. To collect this information, the ISEV Blood EV Task Force created the Minimal Information for Blood EV research (MIBlood-EV), a tool to record and report information about pre-analytical protocols used for plasma and serum preparation as well as assays used to assess the quality of these preparations. This tool does not require modifications of established local pre-analytical protocols and can be easily implemented to enhance existing databases thereby enabling evidence-based optimization of pre-analytical protocols through meta-analysis. Taken together, insight into the quality of prepared plasma and serum samples will (i) improve the quality of biobanks for EV research, (ii) guide the exchange of plasma and serum samples between biobanks and laboratories, (iii) facilitate inter-laboratory comparative EV studies, and (iv) improve the peer review process.
Keywords: biomarker; blood; extracellular vesicles; liquid biopsy; quality control; reproducibility; standardization.
© 2023 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
J.C.J has collaborative research agreements with Beckman Coulter and Cytek Biosciences. R.P. received consulting fees from MetaGuideX. C.L. has collaborative research agreements with Astra Zeneca (Gothenburg, Sweden). E.B. received research funding from STRM Bio, Mitrix Bio, Veralox and Maresin Pharma. P.S. received funding support from the EV Ecosystem for Theranostic Platforms (Business Finland). K.W.W. has a collaborative research agreement with Ionis Pharmaceuticals; has served as an advisor to Exopharm, NeuroDex, Novadip, and ShiftBio; and performs ad hoc consulting on extracellular vesicles and RNA as Kenneth Witwer Consulting. All authors whose names are listed certify that they have no financial or non‐financial conflicts of interest that would have had influence on the subject matter and materials nor recommendations contained within this manuscript.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources