Methylotrophy in the Mire: direct and indirect routes for methane production in thawing permafrost
- PMID: 38063415
- PMCID: PMC10805028
- DOI: 10.1128/msystems.00698-23
Methylotrophy in the Mire: direct and indirect routes for methane production in thawing permafrost
Abstract
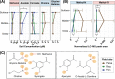
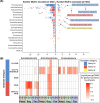
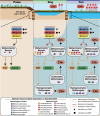
While wetlands are major sources of biogenic methane (CH4), our understanding of resident microbial metabolism is incomplete, which compromises the prediction of CH4 emissions under ongoing climate change. Here, we employed genome-resolved multi-omics to expand our understanding of methanogenesis in the thawing permafrost peatland of Stordalen Mire in Arctic Sweden. In quadrupling the genomic representation of the site's methanogens and examining their encoded metabolism, we revealed that nearly 20% of the metagenome-assembled genomes (MAGs) encoded the potential for methylotrophic methanogenesis. Further, 27% of the transcriptionally active methanogens expressed methylotrophic genes; for Methanosarcinales and Methanobacteriales MAGs, these data indicated the use of methylated oxygen compounds (e.g., methanol), while for Methanomassiliicoccales, they primarily implicated methyl sulfides and methylamines. In addition to methanogenic methylotrophy, >1,700 bacterial MAGs across 19 phyla encoded anaerobic methylotrophic potential, with expression across 12 phyla. Metabolomic analyses revealed the presence of diverse methylated compounds in the Mire, including some known methylotrophic substrates. Active methylotrophy was observed across all stages of a permafrost thaw gradient in Stordalen, with the most frozen non-methanogenic palsa found to host bacterial methylotrophy and the partially thawed bog and fully thawed fen seen to house both methanogenic and bacterial methylotrophic activities. Methanogenesis across increasing permafrost thaw is thus revised from the sole dominance of hydrogenotrophic production and the appearance of acetoclastic at full thaw to consider the co-occurrence of methylotrophy throughout. Collectively, these findings indicate that methanogenic and bacterial methylotrophy may be an important and previously underappreciated component of carbon cycling and emissions in these rapidly changing wetland habitats.IMPORTANCEWetlands are the biggest natural source of atmospheric methane (CH4) emissions, yet we have an incomplete understanding of the suite of microbial metabolism that results in CH4 formation. Specifically, methanogenesis from methylated compounds is excluded from all ecosystem models used to predict wetland contributions to the global CH4 budget. Though recent studies have shown methylotrophic methanogenesis to be active across wetlands, the broad climatic importance of the metabolism remains critically understudied. Further, some methylotrophic bacteria are known to produce methanogenic by-products like acetate, increasing the complexity of the microbial methylotrophic metabolic network. Prior studies of Stordalen Mire have suggested that methylotrophic methanogenesis is irrelevant in situ and have not emphasized the bacterial capacity for metabolism, both of which we countered in this study. The importance of our findings lies in the significant advancement toward unraveling the broader impact of methylotrophs in wetland methanogenesis and, consequently, their contribution to the terrestrial global carbon cycle.
Keywords: EMERGE Biology Integration Institute; Stordalen Mire; methanogenesis; methylotrophy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Saunois M, Jackson RB, Bousquet P, Poulter B, Canadell JG. 2016. The growing role of methane in anthropogenic climate change. Environ Res Lett 11:120207. doi:10.1088/1748-9326/11/12/120207 - DOI
-
- Schuur EAG, McGuire AD, Schädel C, Grosse G, Harden JW, Hayes DJ, Hugelius G, Koven CD, Kuhry P, Lawrence DM, Natali SM, Olefeldt D, Romanovsky VE, Schaefer K, Turetsky MR, Treat CC, Vonk JE. 2015. Climate change and the permafrost carbon feedback. Nature 520:171–179. doi:10.1038/nature14338 - DOI - PubMed
-
- Conrad R. 2020. Importance of hydrogenotrophic, aceticlastic and methylotrophic methanogenesis for methane production in terrestrial, aquatic and other anoxic environments: a mini review. Pedosphere 30:25–39. doi:10.1016/S1002-0160(18)60052-9 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
