TLR9 plus STING Agonist Adjuvant Combination Induces Potent Neopeptide T Cell Immunity and Improves Immune Checkpoint Blockade Efficacy in a Tumor Model
- PMID: 38063488
- PMCID: PMC10784725
- DOI: 10.4049/jimmunol.2300038
TLR9 plus STING Agonist Adjuvant Combination Induces Potent Neopeptide T Cell Immunity and Improves Immune Checkpoint Blockade Efficacy in a Tumor Model
Abstract
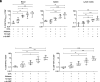
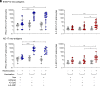
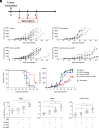
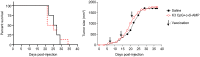
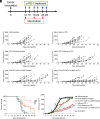
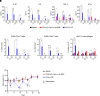
Immune checkpoint blockade (ICB) immunotherapies have emerged as promising strategies for the treatment of cancer; however, there remains a need to improve their efficacy. Determinants of ICB efficacy are the frequency of tumor mutations, the associated neoantigens, and the T cell response against them. Therefore, it is expected that neoantigen vaccinations that boost the antitumor T cell response would improve ICB therapy efficacy. The aim of this study was to develop a highly immunogenic vaccine using pattern recognition receptor agonists in combination with synthetic long peptides to induce potent neoantigen-specific T cell responses. We determined that the combination of the TLR9 agonist K-type CpG oligodeoxynucleotides (K3 CpG) with the STING agonist c-di-AMP (K3/c-di-AMP combination) significantly increased dendritic cell activation. We found that immunizing mice with 20-mer of either an OVA peptide, low-affinity OVA peptides, or neopeptides identified from mouse melanoma or lung mesothelioma, together with K3/c-di-AMP, induced potent Ag-specific T cell responses. The combined K3/c-di-AMP adjuvant formulation induced 10 times higher T cell responses against neopeptides than the TLR3 agonist polyinosinic:polycytidylic acid, a derivative of which is the leading adjuvant in clinical trials of neoantigen peptide vaccines. Moreover, we demonstrated that our K3/c-di-AMP vaccine formulation with 20-mer OVA peptide was capable of controlling tumor growth and improving survival in B16-F10-OVA tumor-bearing C57BL/6 mice and synergized with anti-PD-1 treatment. Together, our findings demonstrate that the K3/c-di-AMP vaccine formulation induces potent T cell immunity against synthetic long peptides and is a promising candidate to improve neoantigen vaccine platform.
Copyright © 2024 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


References
-
- Garon, E. B., Rizvi N. A., Hui R., Leighl N., Balmanoukian A. S., Eder J. P., Patnaik A., Aggarwal C., Gubens M., Horn L., et al. KEYNOTE-001 Investigators . 2015. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372: 2018–2028. - PubMed
-
- Rosenberg, J. E., Hoffman-Censits J., Powles T., van der Heijden M. S., Balar A. V., Necchi A., Dawson N., O’Donnell P. H., Balmanoukian A., Loriot Y., et al. . 2016. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387: 1909–1920. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

