Advanced Flame Spray Pyrolysis (FSP) Technologies for Engineering Multifunctional Nanostructures and Nanodevices
- PMID: 38063702
- PMCID: PMC10707979
- DOI: 10.3390/nano13233006
Advanced Flame Spray Pyrolysis (FSP) Technologies for Engineering Multifunctional Nanostructures and Nanodevices
Abstract
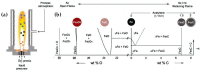
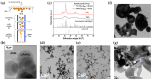
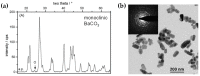
Flame spray pyrolysis (FSP) is an industrially scalable technology that enables the engineering of a wide range of metal-based nanomaterials with tailored properties nanoparticles. In the present review, we discuss the recent state-of-the-art advances in FSP technology with regard to nanostructure engineering as well as the FSP reactor setup designs. The challenges of in situ incorporation of nanoparticles into complex functional arrays are reviewed, underscoring FSP's transformative potential in next-generation nanodevice fabrication. Key areas of focus include the integration of FSP into the technology readiness level (TRL) for nanomaterials production, the FSP process design, and recent advancements in nanodevice development. With a comprehensive overview of engineering methodologies such as the oxygen-deficient process, double-nozzle configuration, and in situ coatings deposition, this review charts the trajectory of FSP from its foundational roots to its contemporary applications in intricate nanostructure and nanodevice synthesis.
Keywords: TRL; complex assemblies; double nozzle; flame spray pyrolysis; multifunctional nanomaterials/nanodevices; nanofilms; non-oxides; oxygen-deficiency process; perovskites; plasmonics; quantum dots.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

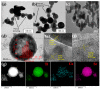






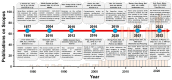

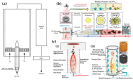


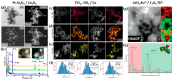
















References
-
- Meierhofer F., Fritsching U. Synthesis of Metal Oxide Nanoparticles in Flame Sprays: Review on Process Technology, Modeling, and Diagnostics. Energy Fuels. 2021;35:5495–5537. doi: 10.1021/acs.energyfuels.0c04054. - DOI
-
- Ulrich G.D. Special Report. Chem. Eng. News Arch. 1984;62:22–29. doi: 10.1021/cen-v062n032.p022. - DOI
-
- Liu S., Mohammadi M.M., Swihart M.T. Fundamentals and Recent Applications of Catalyst Synthesis Using Flame Aerosol Technology. Chem. Eng. J. 2021;405:126958. doi: 10.1016/j.cej.2020.126958. - DOI
-
- Leskelä M., Ritala M. Atomic Layer Deposition (ALD): From Precursors to Thin Film Structures. Thin Solid Films. 2002;409:138–146. doi: 10.1016/S0040-6090(02)00117-7. - DOI
Publication types
LinkOut - more resources
Full Text Sources

