Polypyrrole Solid-State Supercapacitors Drawn on Paper
- PMID: 38063735
- PMCID: PMC10707751
- DOI: 10.3390/nano13233040
Polypyrrole Solid-State Supercapacitors Drawn on Paper
Abstract
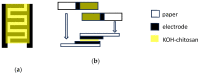
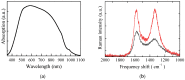
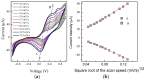
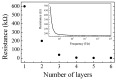
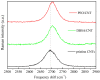
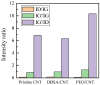
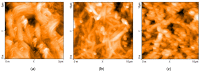
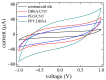
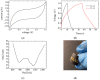
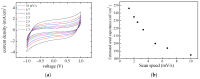
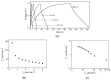
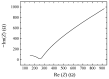
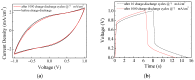
Solid-state supercapacitors with areal capacitance in the order of 100 mF⋅cm-2 are developed on paper substrates, using eco-friendly, low-cost materials and a simple technology. The electrochemically active material used as the electrode is prepared from a stable water-based ink, obtained by doping commercial polypyrrole (PPY) powder with dodecylbenzene sulfonic acid (DBSA), and characterized by optical and electrical measurements, Raman investigation and Atomic Force Microscopy. The PPY:DBSA ink can be directly applied on paper by means of rechargeable water pens, obtaining, after drying, electrically conducting solid state tracks. The PPY:DBSA layers are then interfaced to one another through a polymer gel based on potassium hydroxide and chitosan, acting both as the ion-conducting medium and as the separator. The areal capacitance of the devices developed by following such a simple rule can be improved when the PPY:DBSA ink is applied in combination with other nanostructured carbon material.
Keywords: paper substrates; polypyrrole; supercapacitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Yu X., Chen R., Gan L., Li H., Chen L. Battery Safety: From Lithium-Ion to Solid-State Batteries. Engineering. 2023;21:9–14. doi: 10.1016/j.eng.2022.06.022. - DOI
-
- Doughty D.H., Roth E.P. A General Discussion of Li Ion Battery Safety. Electrochem. Soc. Interface. 2012;21:37.
-
- Held M., Tuchschmid M., Zennegg M., Figi R., Schreiner C., Mellert L.D., Welte U., Kompatscher M., Hermann M., Nachef L. Thermal runaway and fire of electric vehicle lithium-ion battery and contamination of infrastructure facility. Renew. Sustain. Energy Rev. 2022;165:112474–112487. doi: 10.1016/j.rser.2022.112474. - DOI
-
- Melchor-Martínez E.M., Macias-Garbett R., Malacara-Becerra A., Iqbal H.M.N., Sosa-Hernandez J.E., Parra-Saldívar R. Environmental impact of emerging contaminants from battery waste: A mini review. Case Stud. Chem. Environ. Eng. 2021;3:100104–100112. doi: 10.1016/j.cscee.2021.100104. - DOI
-
- Mrozik W., Rajaeifar M.A., Heidrichab O., Christensen P. Environmental impacts, pollution sources and pathways of spent lithium-ion batteries. Energy Environ. Sci. 2021;14:6099–6121. doi: 10.1039/D1EE00691F. - DOI
LinkOut - more resources
Full Text Sources

