Highly Efficient Liquid-Phase Exfoliation of Layered Perovskite-like Titanates HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) into Nanosheets
- PMID: 38063747
- PMCID: PMC10708289
- DOI: 10.3390/nano13233052
Highly Efficient Liquid-Phase Exfoliation of Layered Perovskite-like Titanates HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) into Nanosheets
Abstract
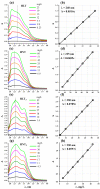
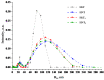
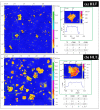
Nanosheets of layered perovskite-like oxides attract researchers as building blocks for the creation of a wide range of demanded nanomaterials. However, Ruddlesden-Popper phases are difficult to separate into nanosheets quantitatively via the conventional liquid-phase exfoliation procedure in aqueous solutions of bulky organic bases. The present study has considered systematically a relatively novel and efficient approach to a high-yield preparation of concentrated suspensions of perovskite nanosheets. For this, the Ruddlesden-Popper titanates HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) have been intercalated by n-alkylamines with various chain lengths, exposed to sonication in aqueous tetrabutylammonium hydroxide (TBAOH) and centrifuged to separate the nanosheet-containing supernatant. The experiments included variations of a wide range of conditions, which allowed for the achievement of impressive nanosheet concentrations in suspensions up to 2.1 g/L and yields up to 95%. The latter were found to strongly depend on the length of intercalated n-alkylamines. Despite the less expanded interlayer space, the titanates modified with short-chain amines demonstrated a much higher completeness of liquid-phase exfoliation as compared to those with long-chain ones. It was also shown that the exfoliation efficiency depends more on the sample stirring time in the TBAOH solution than on the sonication duration. Analysis of the titanate nanosheets obtained by means of dynamic light scattering, electron and atomic force microscopy revealed their lateral sizes of 30-250 nm and thickness of 2-4 nm. The investigated exfoliation strategy appears to be convenient for the high-yield production of perovskite nanosheet-based materials for photocatalytic hydrogen production, environmental remediation and other applications.
Keywords: amine; exfoliation; intercalation; layered perovskite; nanosheets; suspension stability; titanate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Hinterding R., Feldhoff A. Two-Dimensional Oxides: Recent Progress in Nanosheets. Z. Phys. Chem. 2018;233:117–165. doi: 10.1515/zpch-2018-1125. - DOI
-
- Takagaki A., Tagusagawa C., Hayashi S., Hara M., Domen K. Nanosheets as Highly Active Solid Acid Catalysts for Green Chemical Syntheses. Energy Environ. Sci. 2010;3:82–93. doi: 10.1039/B918563A. - DOI
-
- Zhao Y., Zhang S., Shi R., Waterhouse G.I.N., Tang J., Zhang T. Two-Dimensional Photocatalyst Design: A Critical Review of Recent Experimental and Computational Advances. Mater. Today. 2020;34:78–91. doi: 10.1016/j.mattod.2019.10.022. - DOI
-
- Le T.B.N., Chang C.W., Su Y.H. Hydrogen Generation Ability of B-Site Substituted Two-Dimensional Can−1Tin−3Nb3O3n+1− Perovskite Nanosheets in Photoelectrochemical Cell. Surf. Interfaces. 2023;36:102623. doi: 10.1016/j.surfin.2022.102623. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

