Recent Advances in LDH/g-C3N4 Heterojunction Photocatalysts for Organic Pollutant Removal
- PMID: 38063762
- PMCID: PMC10707826
- DOI: 10.3390/nano13233066
Recent Advances in LDH/g-C3N4 Heterojunction Photocatalysts for Organic Pollutant Removal
Abstract
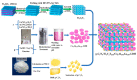
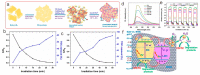
Environmental pollution has been decreased by using photocatalytic technology in conjunction with solar energy. An efficient method to obtain highly efficient photocatalysts is to build heterojunction photocatalysts by combining graphitic carbon nitride (g-C3N4) with layered double hydroxides (LDHs). In this review, recent developments in LDH/g-C3N4 heterojunctions and their applications for organic pollutant removal are systematically exhibited. The advantages of LDH/g-C3N4 heterojunction are first summarized to provide some overall understanding of them. Then, a variety of approaches to successfully assembling LDH and g-C3N4 are simply illustrated. Last but not least, certain unmet research needs for the LDH/g-C3N4 heterojunction are suggested. This review can provide some new insights for the development of high-performance LDH/g-C3N4 heterojunction photocatalysts. It is indisputable that the LDH/g-C3N4 heterojunctions can serve as high-performance photocatalysts to make new progress in organic pollutant removal.
Keywords: carbon nitride; heterojunction; layered double hydroxides; organic pollutant removal; photocatalysis.
Conflict of interest statement
Author Cheng Du, Jialin Xu, Dayong He and Hao Zhang were employed by the company Shenzhen Mindray Bio-Medical Electronics Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Li C., Jia R., Yang Y., Liao G. A Hierarchical Helical Carbon Nanotube Fiber Artificial Ligament. Adv. Fiber Mater. 2023;5:1549–1551. doi: 10.1007/s42765-023-00312-5. - DOI
-
- Lan Q., Jin S.-J., Wang Z., Li X.-Y., Xiong Y., Wang Z.-C., Liu S.-S., Zhang Z.-M., Zhao Q. Design and synthesis of polyoxovanadate-based framework for efficient dye degradation. Tungsten. 2023 doi: 10.1007/s42864-023-00233-1. - DOI
-
- Khan A., Nilam B., Rukhsar C., Sayali G., Mandlekar B., Kadam A. A review article based on composite graphene @tungsten oxide thin films for various applications. Tungsten. 2023;5:391–418. doi: 10.1007/s42864-022-00158-1. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

