Microfluidic Vaterite Synthesis: Approaching the Nanoscale Particles
- PMID: 38063771
- PMCID: PMC10708111
- DOI: 10.3390/nano13233075
Microfluidic Vaterite Synthesis: Approaching the Nanoscale Particles
Abstract
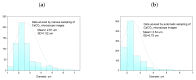
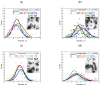
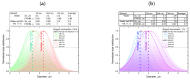
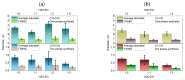
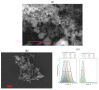
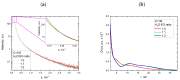
The challenge of continuous CaCO3 particle synthesis is addressed using microfluidic technology. A custom microfluidic chip was used to synthesize CaCO3 nanoparticles in vaterite form. Our focus revolved around exploring one-phase and two-phase synthesis methods tailored for the crystallization of these nanoparticles. The combination of scanning electron microscopy, X-ray diffraction, dynamic light scattering, and small-angle scattering allowed for an evaluation of the synthesis efficiency, including the particle size distribution, morphology, and polymorph composition. The results demonstrated the superior performance of the two-phase system when precipitation occurred inside emulsion microreactors, providing improved size control compared with the one-phase approach. We also discussed insights into particle size changes during the transition from one-phase to two-phase synthesis. The ability to obtain CaCO3 nanoparticles in the desired polymorph form (∼50 nm in size, 86-99% vaterite phase) with the possibility of scaling up the synthesis will open up opportunities for various industrial applications of the developed two-phase microfluidic method.
Keywords: CaCO3; additive manufacturing; microfluidic synthesis; nanoparticles; one-phase synthesis; two-phase synthesis; vaterite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Chen Y., Ji X., Zhao G., Wang X. Facile preparation of cubic calcium carbonate nanoparticles with hydrophobic properties via a carbonation route. Powder Technol. 2010;200:144–148. doi: 10.1016/j.powtec.2010.02.017. - DOI
-
- Haložan D., Riebentanz U., Brumen M., Donath E. Polyelectrolyte microcapsules and coated CaCO3 particles as fluorescence activated sensors in flowmetry. Colloids Surfaces A Physicochem. Eng. Asp. 2009;342:115–121. doi: 10.1016/j.colsurfa.2009.04.024. - DOI
-
- Fujiwara M., Shiokawa K., Morigaki K., Zhu Y., Nakahara Y. Calcium carbonate microcapsules encapsulating biomacromolecules. Chem. Eng. J. 2008;137:14–22. doi: 10.1016/j.cej.2007.09.010. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

