Posttreatment with PaPE-1 Protects from Aβ-Induced Neurodegeneration Through Inhibiting the Expression of Alzheimer's Disease-Related Genes and Apoptosis Process That Involves Enhanced DNA Methylation of Specific Genes
- PMID: 38064105
- PMCID: PMC11236864
- DOI: 10.1007/s12035-023-03819-5
Posttreatment with PaPE-1 Protects from Aβ-Induced Neurodegeneration Through Inhibiting the Expression of Alzheimer's Disease-Related Genes and Apoptosis Process That Involves Enhanced DNA Methylation of Specific Genes
Abstract
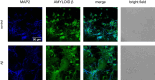
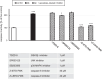
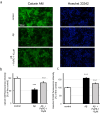
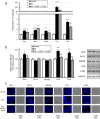
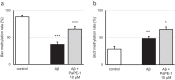
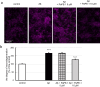
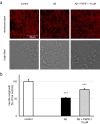
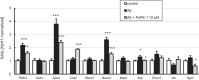
Targeting the non-nuclear estrogen receptor (ER) signaling has been postulated as novel therapeutic strategy for central nervous system pathologies. Recently, we showed that newly designed PaPE-1 (Pathway Preferential Estrogen-1), which selectively activates ER non-nuclear signaling pathways, elicited neuroprotection in a cellular model of Alzheimer's disease (AD) when it was applied at the same time as amyloid-β (Aβ). Since delayed treatment reflects clinical settings better than cotreatment does, current basic study proposes a novel therapeutic approach for AD that relies on a posttreatment with PaPE-1. In this study, mouse neuronal cell cultures treated with preaggregated Aβ1-42 (10 µM) showed the presence of extracellular Aβ1-42, confirming the adequacy of the AD model used. We are the first to demonstrate that a 24-h delayed posttreatment with PaPE-1 decreased the degree of Aβ-induced neurodegeneration, restored neurite outgrowth, and inhibited the expression of AD-related genes, i.e., Rbfox, Apoe, Bace2, App, and Ngrn, except for Chat, which was stimulated. In addition, PaPE-1 elicited anti-apoptotic effects by inhibiting Aβ-induced caspase activities as well as attenuating apoptotic chromatin condensation, and in these ways, PaPE-1 prevented neuronal cell death. Posttreatment with PaPE-1 also downregulated the Aβ-affected mRNA expression of apoptosis-specific factors, such as Bax, Gsk3b, Fas, and Fasl, except for Bcl2, which was upregulated by PaPE-1. In parallel, PaPE-1 decreased the protein levels of BAX, FAS, and FASL, which were elevated in response to Aβ. PaPE-1 elicited a decrease in the BAX/BCL2 ratio that corresponds to increased methylation of the Bax gene. However, the PaPE-1-evoked Bcl2 gene hypermethylation suggests other PaPE-1-dependent mechanisms to control Aβ-induced apoptosis.
Keywords: Alzheimer’s disease; Amyloid-β; Caspases; Neuroprotection; Non-nuclear estrogen receptor signaling; Primary neocortical cell cultures.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA et al (1997) A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem 272(29):17907–17911. 10.1074/jbc.272.29.17907 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

