Development, Physicochemical Characteristics and Pharmacokinetics of a New Sustained-Release Bilayer Tablet Formulation of Tramadol with an Immediate-Release Component for Twice-Daily Administration
- PMID: 38064122
- PMCID: PMC10781817
- DOI: 10.1007/s13318-023-00865-1
Development, Physicochemical Characteristics and Pharmacokinetics of a New Sustained-Release Bilayer Tablet Formulation of Tramadol with an Immediate-Release Component for Twice-Daily Administration
Abstract
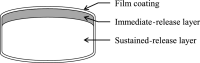
Background and objective: There are some potential concerns about the currently marketed solid oral dosage forms of tramadol, including decreased adherence to immediate-release (IR) formulations due to the high number of doses taken each day and the slow rise in the blood tramadol concentration after administration of sustained-release (SR) formulations, which may not achieve a rapid analgesic effect. To overcome these potential concerns, a twice-daily double-layered tablet formulation of tramadol comprising IR and SR layers was developed. This article reports studies that assessed its physicochemical and pharmacokinetic properties.
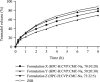
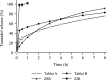
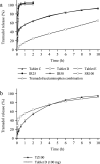
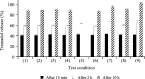
Methods: Dissolution tests of five bilayer tablet formulations (designated tablets A-E) and pharmacokinetic studies of tablets A and B were conducted to investigate the appropriate ratio of the IR/SR layers in the double-layered tablet. Additionally, pharmacokinetic studies of three finished dosage formulations (tablets C-E) were performed in healthy adult males to investigate the effect of food intake on drug absorption.
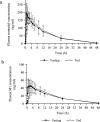
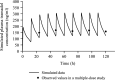
Results: Adjusting the excipients and tramadol content in the IR and SR layers of tablets A-E altered their dissolution profiles in a manner that could be predicted based on their compositions. The IR layer was released within 15 min, and the SR layer was slowly released over 10 h. In the pharmacokinetic study, the time to maximum plasma concentration (tmax) of tramadol after administration of tablets A (IR:SR: 20:80 mg) and B (40:60 mg) was shorter than that of a commercially available SR tablet, and the half-life (t1/2) was longer than that of a commercially available IR tablet. For tablets C-E, administration after food did not affect the area under the concentration-time curve (AUC) or maximum drug concentration (Cmax) of tramadol, but the tmax was prolonged by about 1 h compared with administration in fasting conditions. The mean ± standard deviation tmax and t1/2 for tablet D (IR:SR: 35:65 mg) in the fasting condition was 1.09 ± 0.56 h and 7.82 ± 0.85 h, respectively. The respective values in the fed condition were 2.47 ± 1.06 h and 7.12 ± 0.85 h, respectively.
Conclusions: To address the potential concerns regarding existing formulations of tramadol, a twice-daily, extended-release bilayer formulation of tramadol consisting of an IR and SR layer was developed. Pharmacokinetic studies confirmed that the plasma tramadol concentration increased quickly after administration and was maintained over a long period of time.
© 2023. The Author(s).
Conflict of interest statement
All authors are employees of Nippon Zoki Pharmaceutical Co., Ltd. The authors are also listed as co-inventors on patent applications filed by Nippon Zoki Pharmaceutical Co., Ltd.
Figures


References
-
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/s1474-4422(14)70251-0. - DOI - PMC - PubMed
-
- Japan Society of Pain Clinicians Committee for the Guidelines for the Pharmacologic Management of Neuropathic Pain. Guidelines for the Pharmacologic Management of Neuropathic Pain, 2nd ed, 2016. Tokyo: Shinko Trading Co., Ltd.
-
- Japanese Society for Palliative Medicine. Clinical Guidelines for Cancer Pain Management, 2nd ed, 2020. Tokyo: Kanehara & Co., Ltd.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

