An Autologous Macrophage-Based Phenotypic Transformation-Collagen Degradation System Treating Advanced Liver Fibrosis
- PMID: 38064164
- PMCID: PMC10870050
- DOI: 10.1002/advs.202306899
An Autologous Macrophage-Based Phenotypic Transformation-Collagen Degradation System Treating Advanced Liver Fibrosis
Abstract
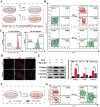
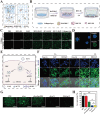
In advanced liver fibrosis (LF), macrophages maintain the inflammatory environment in the liver and accelerate LF deterioration by secreting proinflammatory cytokines. However, there is still no effective strategy to regulate macrophages because of the difficulty and complexity of macrophage inflammatory phenotypic modulation and the insufficient therapeutic efficacy caused by the extracellular matrix (ECM) barrier. Here, AC73 and siUSP1 dual drug-loaded lipid nanoparticle is designed to carry milk fat globule epidermal growth factor 8 (MFG-E8) (named MUA/Y) to effectively inhibit macrophage proinflammatory signals and degrade the ECM barrier. MFG-E8 is released in response to the high reactive oxygen species (ROS) environment in LF, transforming macrophages from a proinflammatory (M1) to an anti-inflammatory (M2) phenotype and inducing macrophages to phagocytose collagen. Collagen ablation increases AC73 and siUSP1 accumulation in hepatic stellate cells (HSCs) and inhibits HSCs overactivation. Interestingly, complete resolution of liver inflammation, significant collagen degradation, and HSCs deactivation are observed in methionine choline deficiency (MCD) and CCl4 models after tail vein injection of MUA/Y. Overall, this work reveals a macrophage-focused regulatory treatment strategy to eliminate LF progression at the source, providing a new perspective for the clinical treatment of advanced LF.
Keywords: advanced hepatic fibrosis; hepatic stellate cells; in situ collagen degradation; inflammation; lipid nanoparticle; macrophages.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Shetty S., Patten D., Rantakari P., Weston C., Jalkanen S., Salmi M., Adams D., Lancet 2017, 389, S15.
-
- Reig M., Cabibbo G., J. Hepatol. 2021, 74, 1225. - PubMed
-
- Fan R., Li G., Yu N., Chang X., Arshad T., Liu W.‐Y., Chen Y., Wong G. L.‐H., Jiang Y., Liang X., Chen Y., Jin X.‐Z., Dong Z., Leung H. H.‐W., Wang X.‐D., Zeng Z., Yip T. C.‐F., Xie Q., Tan D., You S., Ji D., Zhao J., Sanyal A. J., Sun J., Zheng M.‐H., Wong V. W.‐S., Yang Y., Hou J., Clin. Gastroenterol. Hepatol. 2023, 21, 3070. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2022YFE0198400/National Key R&D Program of China
- 82020108029/National Natural Science Foundation of China
- 82073398/National Natural Science Foundation of China
- SKLNMZZ202021/Project of State Key Laboratory of Natural Medicines, China Pharmaceutical University
- CPU2018GY06/Double First-class University Projects
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
