MMP-2 regulates Src activation via repression of the CHK/MATK tumor suppressor in osteosarcoma
- PMID: 38064181
- PMCID: PMC10849928
- DOI: 10.1002/cnr2.1946
MMP-2 regulates Src activation via repression of the CHK/MATK tumor suppressor in osteosarcoma
Abstract
Background: Doxorubicin, a first-line anticancer drug for osteosarcoma treatment, has been the subject of recent research exploring the mechanisms behind its chemoresistance and its ability to enhance cell migration at sublethal concentrations. Matrix metalloproteinase-2 (MMP-2), a type IV collagenase and zinc-dependent endopeptidase, is well-known for degrading the extracellular matrix and promoting cancer metastasis. Our previous work demonstrated that nuclear MMP-2 regulates ribosomal RNA transcription via histone clipping, thereby controlling gene expression. Additionally, MMP-2 activity is regulated by the non-receptor tyrosine kinase and oncogene, Src, which plays a crucial role in cell adhesion, invasion, and metastasis. Src kinase is primarily regulated by two endogenous inhibitors: C-terminal Src kinase (Csk) and Csk homologous kinase (CHK/MATK).
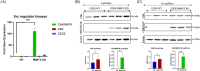
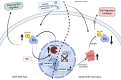
Aim: In this study, we reveal that the MMP-2 gene acts as an upstream regulator of Src kinase activity by suppressing its endogenous inhibitor, CHK/MATK, in osteosarcoma cells.
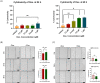
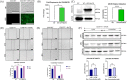
Methods and results: We show that enhanced osteosarcoma cell migration which is induced by sublethal concentrations of doxorubicin can be overcome by inactivating the MMP-2 gene or overexpressing CHK/MATK. Our findings highlight the MMP-2 gene as a promising additional target for combating cancer cell migration and metastasis. This is due to its role in suppressing on the gene and protein expression of the tumor suppressor CHK/MATK in osteosarcoma.
Conclusion: By targeting the MMP-2 gene, we can potentially enhance the effectiveness of doxorubicin treatment and reduce chemoresistance in osteosarcoma.
Keywords: Csk homologous kinase (CHK/MATK); Src; Src family kinases (SFK); doxorubicin; matrix metalloproteinase-2 (MMP-2); osteosarcoma.
© 2023 The Authors. Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Figures


References
-
- Li H, Qiu Z, Li F, Wang C. The relationship between MMP‐2 and MMP‐9 expression levels with breast cancer incidence and prognosis. Oncol Lett [Internet]. 2017;14:5865‐5870. http://www.spandidos-publications.com/10.3892/ol.2017.6924 - DOI - PMC - PubMed
-
- Xu X, Wang Y, Chen Z, Sternlicht MD, Hidalgo M, Steffensen B. Matrix metalloproteinase‐2 contributes to cancer cell migration on collagen. Cancer Res. 2005;65(1):130‐136. - PubMed
-
- Pavlaki M, Zucker S. Matrix metalloproteinase inhibitors (MMPIs): the beginning of phase I or the termination of phase III clinical trials. Cancer Metastasis Rev. 2003;22(2–3):177‐203. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

