The role of ACT score in mepolizumab discontinuation
- PMID: 38064231
- PMCID: PMC11076164
- DOI: 10.1080/02770903.2023.2293067
The role of ACT score in mepolizumab discontinuation
Abstract
Background: Mepolizumab is a therapy for severe asthma. We have little knowledge of the characteristics of people in the US that discontinue mepolizumab in clinical care.
Objective: To investigate the real-world efficacy and time to clinical discontinuation of mepolizumab, we evaluated individuals with asthma started on mepolizumab at the Cleveland Clinic. We hypothesized that individuals that discontinue mepolizumab have more severe and uncontrolled asthma at baseline.
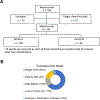
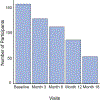
Methods: Between 2016 and 2022, patients who started on mepolizumab consented to be assessed over 18 months. At baseline, a questionnaire including demographic and medical history was collected. Laboratory findings such as ACT score, FENO (Fractional Excretion of Nitric Oxide), and spirometry were recorded. At the conclusion of the observation period, the participants were divided into two categories: Group A and Group B.
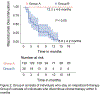
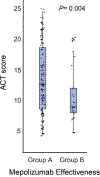
Results: Group B [N = 28] discontinued mepolizumab (p < 0.05) at an average of 5.8 months (SD 4.2 months). Group A [N = 129] stayed on the therapy for at least 1 year. A participant with an ACT score less than 13 has an odds ratio of 6.64 (95% CI, 2.1 - 26.0) of discontinuing mepolizumab therapy. For a male, the odds of discontinuing mepolizumab therapy is 3.39 (95% CI, 1.1-11.2).
Conclusion: In this real-world study, we find that high eosinophil count may not be adequate in screening which individuals will benefit from mepolizumab. Up to 17% of patients fail therapy within 6 months, with male sex and low ACT score increasing risk of mepolizumab discontinuation at Cleveland Clinic.
Keywords: Asthma; asthma control; mepolizumab.
Figures





References
-
- Venkatesan P 2023 GINA report for asthma. Lancet Respir Med. 2023. - PubMed
-
- Reibman J, Tan L, Ambrose C, Chung Y, Desai P, Llanos JP, et al. Clinical and economic burden of severe asthma among US patients treated with biologic therapies. Ann Allergy Asthma Immunol. 2021;127(3):318–25 e2. - PubMed
-
- Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC, Menzies-Gow AN, et al. Characterization of Severe Asthma Worldwide: Data From the International Severe Asthma Registry. Chest. 2020;157(4):790–804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
