Predictive value of immunotherapy-induced inflammation indexes: dynamic changes in patients with nasopharyngeal carcinoma receiving immune checkpoint inhibitors
- PMID: 38065623
- PMCID: PMC10836292
- DOI: 10.1080/07853890.2023.2280002
Predictive value of immunotherapy-induced inflammation indexes: dynamic changes in patients with nasopharyngeal carcinoma receiving immune checkpoint inhibitors
Abstract
Background: Immune checkpoint inhibitors (ICIs) have achieved substantial advancements in clinical care. However, there is no strong evidence for identified biomarkers of ICIs in NPC.
Methods: In this retrospective study, 284 patients were enrolled into a training or validation cohort. Inflammatory indexes based on peripheral blood parameters were evaluated, including the systemic immune-inflammation index (SII), the neutrophil-lymphocyte ratio (NLR), the platelet-lymphocyte ratio (PLR), the lymphocyte-to-C-reactive protein ratio (LCR), and the lymphocyte-monocyte ratio (LMR). The optimum cut-off value for patient stratification was identified using X-tile. The Kaplan-Meier method and Cox's proportional regression analyses were used to identify prognostic factors.
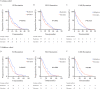
Results: Immunotherapy significantly changed the levels of SII, NLR, PLR, LCR and LMR in NPC patients. Patients with lower SII, NLR, and PLR, as well as those with higher LCR and LMR, before immunotherapy had superior PFS (all p < 0.05). Moreover, PFS in the decreased SII, reduced NLR and increased LMR group was significantly longer than in the opposite group (all p < 0.05). Both univariate and multivariate analyses validated that baseline SII and LMR, and the immunotherapy-related SII reduction and LMR elevation were independent prognostic factors for PFS in advanced NPC patients receiving ICIs.
Conclusions: Immune checkpoint inhibitor treatments significantly changed the levels of SII, NLR, PLR, LCR and LMR in NPC patients treated with immunotherapy. A lower baseline SII and a higher baseline LMR, and a reduction in SII and an elevation in LMR after immunotherapy are favorable factors for predicting survival among advanced NPC patients.
Keywords: NPC; immune checkpoint inhibitors; inflammatory and immune-based prognostic indexes; prognosis.
Plain language summary
There is no strong evidence for identified biomarkers of immune checkpoint inhibitors (ICIs) in nasopharyngeal carcinoma (NPC).Lower baseline SII and higher baseline LMR were related to better PFS. The dynamic changes of SII and LMR were independent prognostic factors for the survival of NPC patients receiving ICIs.Neutrophils, platelets, lymphocytes, and monocytes can be used as cheap and valuable biomarkers for predicting tumor response in NPC on immunotherapy.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
Prognostic value of inflammatory markers NLR, PLR, and LMR in gastric cancer patients treated with immune checkpoint inhibitors: a meta-analysis and systematic review.Front Immunol. 2024 Jul 10;15:1408700. doi: 10.3389/fimmu.2024.1408700. eCollection 2024. Front Immunol. 2024. PMID: 39050856 Free PMC article.
-
Prognostic value of inflammatory markers NLR, PLR, LMR, dNLR, ANC in melanoma patients treated with immune checkpoint inhibitors: a meta-analysis and systematic review.Front Immunol. 2024 Oct 18;15:1482746. doi: 10.3389/fimmu.2024.1482746. eCollection 2024. Front Immunol. 2024. PMID: 39493767 Free PMC article.
-
High Expression of NLR and SII in patients With Nasopharyngeal Carcinoma as Potential Prognostic Observations.Cancer Control. 2024 Jan-Dec;31:10732748241288106. doi: 10.1177/10732748241288106. Cancer Control. 2024. PMID: 39323032 Free PMC article.
-
[Relationship between preoperative inflammatory indexes and prognosis of patients with rectal cancer and establishment of prognostic nomogram prediction model].Zhonghua Zhong Liu Za Zhi. 2022 May 23;44(5):402-409. doi: 10.3760/cma.j.cn112152-20200630-00612. Zhonghua Zhong Liu Za Zhi. 2022. PMID: 35615796 Chinese.
-
Systemic immune-inflammatory index predict short-term outcome in recurrent/metastatic and locally advanced cervical cancer patients treated with PD-1 inhibitor.Sci Rep. 2024 Dec 28;14(1):31528. doi: 10.1038/s41598-024-82976-6. Sci Rep. 2024. PMID: 39732889 Free PMC article.
Cited by
-
Monocyte-related markers as predictors of immune checkpoint inhibitor efficacy and immune-related adverse events: a systematic review and meta-analysis.Cancer Metastasis Rev. 2025 Feb 21;44(1):35. doi: 10.1007/s10555-025-10246-6. Cancer Metastasis Rev. 2025. PMID: 39982537 Free PMC article.
-
Platelet-to-Neutrophil Ratio: A Novel Prognostic Indicator for Anti-PD-1-Based Therapy in Relapsed/Refractory Hodgkin Lymphoma and Solid Tumors.MedComm (2020). 2025 May 16;6(6):e70199. doi: 10.1002/mco2.70199. eCollection 2025 Jun. MedComm (2020). 2025. PMID: 40384985 Free PMC article.
-
The impact of preoperative immunonutritional status on prognosis in ovarian cancer: a multicenter real-world study.J Ovarian Res. 2025 Feb 17;18(1):30. doi: 10.1186/s13048-025-01607-4. J Ovarian Res. 2025. PMID: 39962572 Free PMC article.
-
Inflammatory markers predict efficacy of immunotherapy in advanced non-small cell lung cancer: a preliminary exploratory study.Discov Oncol. 2025 Jan 4;16(1):8. doi: 10.1007/s12672-025-01753-7. Discov Oncol. 2025. PMID: 39755866 Free PMC article.
-
Development and validation of a prognostic nomogram incorporating neutrophil-to-albumin ratio for predicting overall survival in patients with nasopharyngeal carcinoma undergoing concurrent chemoradiotherapy.Heliyon. 2024 Dec 3;11(1):e40881. doi: 10.1016/j.heliyon.2024.e40881. eCollection 2025 Jan 15. Heliyon. 2024. PMID: 39801974 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
