Airway environment drives the selection of quorum sensing mutants and promote Staphylococcus aureus chronic lifestyle
- PMID: 38065959
- PMCID: PMC10709412
- DOI: 10.1038/s41467-023-43863-2
Airway environment drives the selection of quorum sensing mutants and promote Staphylococcus aureus chronic lifestyle
Abstract
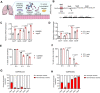
Staphylococcus aureus is a predominant cause of chronic lung infections. While the airway environment is rich in highly sialylated mucins, the interaction of S. aureus with sialic acid is poorly characterized. Using S. aureus USA300 as well as clinical isolates, we demonstrate that quorum-sensing dysfunction, a hallmark of S. aureus adaptation, correlates with a greater ability to consume free sialic acid, providing a growth advantage in an air-liquid interface model and in vivo. Furthermore, RNA-seq experiment reveals that free sialic acid triggers transcriptional reprogramming promoting S. aureus chronic lifestyle. To support the clinical relevance of our results, we show the co-occurrence of S. aureus, sialidase-producing microbiota and free sialic acid in the airway of patients with cystic fibrosis. Our findings suggest a dual role for sialic acid in S. aureus airway infection, triggering virulence reprogramming and driving S. aureus adaptive strategies through the selection of quorum-sensing dysfunctional strains.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Foundation, C. F. CF Registry: Annual Data Report. (2020).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- ANR-21-CE17-0012-01/Agence Nationale de la Recherche (French National Research Agency)
- 201904910463/CSC | Chinese Government Scholarship
- 202106300010/CSC | Chinese Government Scholarship
- ERC-2018-StG-804135/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
LinkOut - more resources
Full Text Sources
Medical

