Cotadutide promotes glycogenolysis in people with overweight or obesity diagnosed with type 2 diabetes
- PMID: 38066113
- PMCID: PMC10730390
- DOI: 10.1038/s42255-023-00938-0
Cotadutide promotes glycogenolysis in people with overweight or obesity diagnosed with type 2 diabetes
Abstract
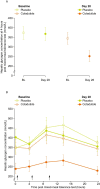
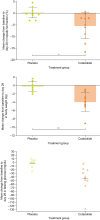
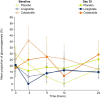
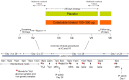
Cotadutide is a dual glucagon-like peptide 1 and glucagon receptor agonist under development for the treatment of non-alcoholic steatohepatitis and type 2 diabetes mellitus (T2DM) and chronic kidney disease. Non-alcoholic steatohepatitis is a complex disease with no approved pharmacotherapies, arising from an underlying state of systemic metabolic dysfunction in association with T2DM and obesity. Cotadutide has been shown to improve glycaemic control, body weight, lipids, liver fat, inflammation and fibrosis. We conducted a two-part, randomized phase 2a trial in men and women with overweight or obesity diagnosed with T2DM to evaluate the efficacy and safety of cotadutide compared with placebo and liraglutide. The primary endpoints were change from baseline to day 28 of treatment in postprandial hepatic glycogen (part A) and to day 35 of treatment in fasting hepatic glycogen (part B) with cotadutide versus placebo. Secondary endpoints in part B were changes in fasting hepatic glycogen with cotadutide versus the mono glucagon-like peptide 1 receptor agonist, liraglutide, and change in hepatic fat fraction. The trial met its primary endpoint. We showed that cotadutide promotes greater reductions in liver glycogen and fat compared with placebo and liraglutide. Safety and tolerability findings with cotadutide were comparable to those of previous reports. Thus, this work provides evidence of additional benefits of cotadutide that could be attributed to glucagon receptor engagement. Our results suggest that cotadutide acts on the glucagon receptor in the human liver to promote glycogenolysis and improve the metabolic health of the liver. ClinicalTrials.gov registration: NCT03555994 .
© 2023. The Author(s).
Conflict of interest statement
This study was funded by AstraZeneca. V.B.S.-H. was supported by a European Research Council starting grant (no. 759161, MRS in Diabetes). Medical writing support was provided by C. Foster, Oxford PharmaGenesis, which was funded by AstraZeneca. V.E.R.P., D.R., Y.-T.C., R.E., L.H., P.A. and L. Jermutus are employees and stockholders of AstraZeneca. E.J. is an employee of Antaros Medical. L. Johansson is an employee and stockholder of Antaros Medical. P.S. has received institutional research funding from AstraZeneca. The other authors declare no conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

