Longitudinal modeling of human neuronal aging reveals the contribution of the RCAN1-TFEB pathway to Huntington's disease neurodegeneration
- PMID: 38066314
- PMCID: PMC11456361
- DOI: 10.1038/s43587-023-00538-3
Longitudinal modeling of human neuronal aging reveals the contribution of the RCAN1-TFEB pathway to Huntington's disease neurodegeneration
Abstract
Aging is a common risk factor in neurodegenerative disorders. Investigating neuronal aging in an isogenic background stands to facilitate analysis of the interplay between neuronal aging and neurodegeneration. Here we perform direct neuronal reprogramming of longitudinally collected human fibroblasts to reveal genetic pathways altered at different ages. Comparative transcriptome analysis of longitudinally aged striatal medium spiny neurons (MSNs) in Huntington's disease identified pathways involving RCAN1, a negative regulator of calcineurin. Notably, RCAN1 protein increased with age in reprogrammed MSNs as well as in human postmortem striatum and RCAN1 knockdown rescued patient-derived MSNs of Huntington's disease from degeneration. RCAN1 knockdown enhanced chromatin accessibility of genes involved in longevity and autophagy, mediated through enhanced calcineurin activity, leading to TFEB's nuclear localization by dephosphorylation. Furthermore, G2-115, an analog of glibenclamide with autophagy-enhancing activities, reduced the RCAN1-calcineurin interaction, phenocopying the effect of RCAN1 knockdown. Our results demonstrate that targeting RCAN1 genetically or pharmacologically can increase neuronal resilience in Huntington's disease.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



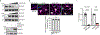



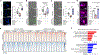



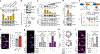
Update of
-
Longitudinal modeling of human neuronal aging identifies RCAN1-TFEB pathway contributing to neurodegeneration of Huntington's disease.Res Sq [Preprint]. 2023 May 9:rs.3.rs-2815300. doi: 10.21203/rs.3.rs-2815300/v1. Res Sq. 2023. Update in: Nat Aging. 2024 Jan;4(1):95-109. doi: 10.1038/s43587-023-00538-3. PMID: 37214956 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

