Lonicerin promotes wound healing in diabetic rats by enhancing blood vessel regeneration through Sirt1-mediated autophagy
- PMID: 38066346
- PMCID: PMC10943091
- DOI: 10.1038/s41401-023-01193-5
Lonicerin promotes wound healing in diabetic rats by enhancing blood vessel regeneration through Sirt1-mediated autophagy
Abstract
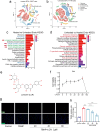
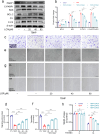
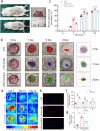
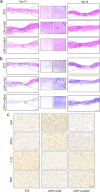
Among the numerous complications of diabetes mellitus, diabetic wounds seriously affect patients' quality of life and result in considerable psychological distress. Promoting blood vessel regeneration in wounds is a crucial step in wound healing. Lonicerin (LCR), a bioactive compound found in plants of the Lonicera japonica species and other honeysuckle plants, exhibits anti-inflammatory and antioxidant activities, and it recently has been found to alleviate ulcerative colitis by enhancing autophagy. In this study we investigated the efficacy of LCR in treatment of diabetic wounds and the underlying mechanisms. By comparing the single-cell transcriptomic data from healing and non-healing states in diabetic foot ulcers (DFU) of 5 patients, we found that autophagy and SIRT signaling activation played a crucial role in mitigating inflammation and oxidative stress, and promoting cell survival in wound healing processes. In TBHP-treated human umbilical vein endothelial cells (HUVECs), we showed that LCR alleviated cell apoptosis, and enhanced the cell viability, migration and angiogenesis. Furthermore, we demonstrated that LCR treatment dose-dependently promoted autophagy in TBHP-treated HUVECs by upregulating Sirt1 expression, and exerted its anti-apoptotic effect through the Sirt1-autophagy axis. Knockdown of Sirt1 significantly decreased the level of autophagy, and mitigated the anti-apoptotic effect of LCR. In a STZ-induced diabetic rat model, administration of LCR significantly promoted wound healing, which was significantly attenuated by Sirt1 knockdown. This study highlights the potential of LCR as a therapeutic agent for the treatment of diabetic wounds and provides insights into the molecular mechanisms underlying its effects.
Keywords: Sirt1; angiogenesis; autophagy; diabetic wounds; lonicerin; oxidative stress; wound healing.
© 2023. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

