Comparison of pregnancy and neonatal outcomes in a retrospective full pregnancy history survey versus population-based prospective records: a validation study in rural Sarlahi District, Nepal
- PMID: 38066542
- PMCID: PMC10709973
- DOI: 10.1186/s41043-023-00472-5
Comparison of pregnancy and neonatal outcomes in a retrospective full pregnancy history survey versus population-based prospective records: a validation study in rural Sarlahi District, Nepal
Abstract
Introduction: Countries without complete civil registration and vital statistics systems rely on retrospective full pregnancy history surveys (FPH) to estimate incidence of pregnancy and mortality outcomes, including stillbirth and neonatal death. Yet surveys are subject to biases that impact demographic estimates, and few studies have quantified these effects. We compare data from an FPH vs. prospective records from a population-based cohort to estimate validity for maternal recall of live births, stillbirths, and neonatal deaths in a rural population in Sarlahi District, Nepal.
Methods: We used prospective data, collected through frequent visits of women from early pregnancy through the neonatal period, from a population-based randomized trial spanning 2010-2017. We randomly selected 76 trial participants from three pregnancy outcome groups: live birth (n = 26), stillbirth (n = 25), or neonatal death (n = 25). Data collectors administered the Nepal 2016 Demographic and Health Surveys (DHS)-VII pregnancy history survey between October 22, 2021, and November 18, 2021. We compared total pregnancy outcomes and numbers of pregnancy and neonatal outcomes between the two data sources. We matched pregnancy outcomes dates in the two sources within ± 30 days and calculated measures of validity for adverse outcomes.
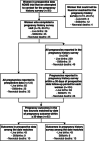
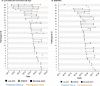
Results: Among 76 participants, we recorded 122 pregnancy outcomes in the prospective data and 104 outcomes in the FPH within ± 30 days of each woman's total observation period in the trial. Among 226 outcomes, we observed 65 live births that survived to 28 days, 25 stillbirths, and 32 live births followed by neonatal death in the prospective data and participants reported 63 live births that survived to 28 days, 15 stillbirths, and 26 live births followed by neonatal death in the pregnancy history survey. Sixty-two FPH outcomes were matched by date within ± 30 days to an outcome in prospective data. Stillbirth, neonatal death, higher parity, and delivery at a health facility were associated with likelihood of a non-matched pregnancy outcome.
Conclusions: Stillbirth and neonatal deaths were underestimated overall by the FPH, potentially underestimating the burden of mortality in this population. There is a need to develop tools to reduce or adjust for biases and errors in retrospective surveys to improve reporting of pregnancy and mortality outcomes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- United Nations Interagency Group for Child Mortality Estimation (UN IGME). Levels and trends in child mortality. New York; 2021.
-
- World Health Organization . Every Newborn: an action plan to end preventable deaths. Geneva: Switzerland; 2014.
-
- UN General Assembly. Transforming our world: the 2030 Agenda for Sustainable Development. 2015 [Available from: https://sustainabledevelopment.un.org/post2015/transformingourworld.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

