The safety and efficacy of tislelizumab, alone or in combination with chemotherapy, for the treatment of non-small cell lung cancer: a systematic review of clinical trials
- PMID: 38066549
- PMCID: PMC10704633
- DOI: 10.1186/s12890-023-02755-3
The safety and efficacy of tislelizumab, alone or in combination with chemotherapy, for the treatment of non-small cell lung cancer: a systematic review of clinical trials
Abstract
Background: Tislelizumab is an anti-programmed death-1 (PD-1) monoclonal antibody with a construction that enables it to have a higher affinity to its target. We aimed to evaluate tislelizumab's safety and efficacy for treating non-small cell lung cancer (NSCLC).
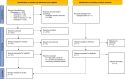
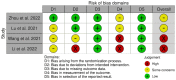
Methods: Embase, Scopus, PubMed, Web of Science, and Google Scholar were searched up to December 20, 2022. The review only included randomized controlled trials (RCTs) that evaluated the safety or efficacy of tislelizumab for treating patients with lung cancer. The revised Cochrane risk-of-bias tool (RoB2) was utilized to evaluate study quality.
Results: There were four RCTs identified, which included 1565 patients with confirmed locally advanced or metastatic squamous and/or non-squamous types of NSCLC. Treatment with tislelizumab was associated with better progression-free survival (PFS) and objective response rate (ORR), particularly when used in combination with chemotherapy. Almost all patients in both arms reported at least one treatment-emergent adverse event (TEAE). Decreased hematologic indexes accounted for more than 20% of the grade ≥ 3 TEAEs in the tislelizumab plus chemotherapy group. The proportion of TEAE that led to death in the tislelizumab plus chemotherapy arms ranged from 3.2 to 4.2%. Hypothyroidism, pneumonitis, and hyperglycemia were the most frequently noted immune-mediated adverse events in the tislelizumab group.
Conclusions: Tislelizumab, whether used alone or in combination with chemotherapy, seems to demonstrate both a safety and efficacy as a treatment for NSCLC.
Keywords: Anti-PD-1 monoclonal antibody; Immune checkpoint inhibitors; Lung Cancer; NSCLC; Systematic review; Tislelizumab.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
No conflict of interest declared.
Figures
References
-
- Alam K, Rahman M, Beg S, Chauhan D, Hafeez A, Almalki WH, et al. et al. Chapter 5 - Advancement in protein-based nanocarriers in targeted anticancer therapy. In: Rahman M, Beg S, Almalki WH, Alhakamy NA, Choudhry H, et al.et al., editors. Nanotherapeutics in Cancer Vaccination. and Challenges: Academic Press; 2022. pp. 95–102.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical