Safety and efficacy of a novel anti-CD19 chimeric antigen receptor T cell product targeting a membrane-proximal domain of CD19 with fast on- and off-rates against non-Hodgkin lymphoma: a first-in-human study
- PMID: 38066564
- PMCID: PMC10709913
- DOI: 10.1186/s12943-023-01886-9
Safety and efficacy of a novel anti-CD19 chimeric antigen receptor T cell product targeting a membrane-proximal domain of CD19 with fast on- and off-rates against non-Hodgkin lymphoma: a first-in-human study
Abstract
Background: Commercial anti-CD19 chimeric antigen receptor T-cell therapies (CART19) are efficacious against advanced B-cell non-Hodgkin lymphoma (NHL); however, most patients ultimately relapse. Several mechanisms contribute to this failure, including CD19-negative escape and CAR T dysfunction. All four commercial CART19 products utilize the FMC63 single-chain variable fragment (scFv) specific to a CD19 membrane-distal epitope and characterized by slow association (on) and dissociation (off) rates. We hypothesized that a novel anti-CD19 scFv that engages an alternative CD19 membrane-proximal epitope independent of FMC63 and that is characterized by faster on- and off-rates could mitigate CART19 failure and improve clinical efficacy.
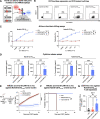
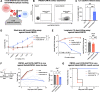
Methods: We developed an autologous CART19 product with 4-1BB co-stimulation using a novel humanized chicken antibody (h1218). This antibody is specific to a membrane-proximal CD19 epitope and harbors faster on/off rates compared to FMC63. We tested h1218-CART19 in vitro and in vivo using FMC63-CART19-resistant models. We conducted a first-in-human multi-center phase I clinical trial to test AT101 (clinical-grade h1218-CART19) in patients with relapsed or refractory (r/r) NHL.
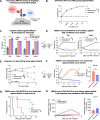
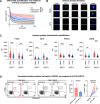
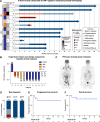
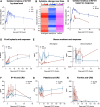
Results: Preclinically, h1218- but not FMC63-CART19 were able to effectively eradicate lymphomas expressing CD19 point mutations (L174V and R163L) or co-expressing FMC63-CAR19 as found in patients relapsing after FMC63-CART19. Furthermore, h1218-CART19 exhibited enhanced killing of B-cell malignancies in vitro and in vivo compared with FMC63-CART19. Mechanistically, we found that h1218-CART19 had reduced activation-induced cell death (AICD) and enhanced expansion compared to FMC63-CART19 owing to faster on- and off-rates. Based on these preclinical results, we performed a phase I dose-escalation trial, testing three dose levels (DL) of AT101 (the GMP version of h1218) using a 3 + 3 design. In 12 treated patients (7 DLBCL, 3 FL, 1 MCL, and 1 MZL), AT101 showed a promising safety profile with 8.3% grade 3 CRS (n = 1) and 8.3% grade 4 ICANS (n = 1). In the whole cohort, the overall response rate was 91.7%, with a complete response rate of 75.0%, which improved to 100% in DL-2 and -3. AT101 expansion correlates with CR and B-cell aplasia.
Conclusions: We developed a novel, safe, and potent CART19 product that recognizes a membrane-proximal domain of CD19 with fast on- and off-rates and showed significant efficacy and promising safety in patients with relapsed B-cell NHL.
Trial registration: NCT05338931; Date: 2022-04-01.
Keywords: CAR T cells; CD19; CD19 mutations; Epitope masking; Fast on- and off-rate; Leukemia; Low avidity; Lymphoma; Membrane-proximal epitope; Resistance.
© 2023. The Author(s).
Conflict of interest statement
MR holds patents related to CD19 CAR T cells (non-AT101) which are licensed to Novartis, Tmunity (Kite-Gilead), and viTToria Biotherapeutics. MR has served as a consultant for NanoString, BMS, GSK, GLG, Sana, Bayer, Guidepoint, Scailyte, and AbClon. MR receives research funding from AbClon, Beckman Coulter, CurioX, Oxoford Nano Imaging, and viTToria Biotherapeutics. MR is the scientific founder of viTToria biotherapeutics. RPP and GG received honoraria for consultation with viTToria Biotherapeutics. DHY has served as a consultant for Roche, Janssen, Amgen, BMS, Novartis, AbClon, GI cell, GC cell, and Pharos Bio. DHY receives honoraria from Roche, Janssen, Amgen, BMS, Kirin, Boryung, and Takeda. DHY receives research funding from Samyang, Kirin, Roche, Janssen and Boryung.
Figures

References
-
- Schuster SJ, Tam CS, Borchmann P, Worel N, McGuirk JP, Holte H, Waller EK, Jaglowski S, Bishop MR, Damon LE, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22:1403–1415. doi: 10.1016/S1470-2045(21)00375-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

