Cohort Profile: International Collaboration on Hepatitis C Elimination in HIV Cohorts (InCHEHC)
- PMID: 38066671
- PMCID: PMC10859136
- DOI: 10.1093/ije/dyad154
Cohort Profile: International Collaboration on Hepatitis C Elimination in HIV Cohorts (InCHEHC)
Keywords: HIV; Hepatitis C; cohort studies; consortium; elimination.
Conflict of interest statement
J.B. reports honoraria for advice or public speaking from Abbvie, Gilead, MSD, Janssen and ViiV Healthcare; and grants from Abbvie, Gilead, MSD and ViiV Healthcare. M.K. reports grants for investigator-initiated studies from ViiV Healthcare, AbbVie, Merck and Gilead, and consulting fees from ViiV Healthcare, AbbVie and Gilead, all outside the submitted work; is supported by a Tier I Canada Research Chair. A.R. reports support to his institution for advisory boards and/or travel grants from Abbvie, MSD, Gilead Sciences and Pfizer; and an investigator-initiated trial (IIT) grant from Gilead Sciences. All remuneration to A.R. went to his home institution and not to A.R. personally, and all remuneration was provided outside the submitted work. K.L. reports honoraria for advice or public speaking from Abbvie, Gilead, MSD, Janssen and ViiH Healthcare. F.B. reports grants from Gilead and honoraria from Gilead, ViiV Healthcare and MSD. M.P. reports unrestricted research grants and speaker/adviser fees from Gilead Sciences and MSD, all of which were paid to her institution and unrelated to the current work. M.vV. reports unrestricted research grants from Gilead and MSD; and fees for participation in advisory boards from Gilead, MSD and ViiV (all paid to his institution). JSD reports funding to his institution for investigator-initiated research from Gilead Sciences and Abbvie; and honoraria to his institution for educational events from AbbVie. L.W. reports grants/financial support for the work under consideration from the French Agency ANRS Emerging Infectious Diseases (ANRS—MIE), paid to her institution. G.M. reports grants from Gilead, Abbvie and ViiV, all paid to her institution and financial support for participating in advisory board from Gilead and ViiV. The CEASE study is supported by Gilead. I.J. reports grants from MSD and ViiV Healthcare, all paid to her institution; honoraria for lectures/presentations from Gilead and ViiV Healthcare; support from Gilead for attending meetings/travel; and support from Gilead to participate in an advisory board. M.H. reports investigator-initiated research grants from Gilead and Abbvie. D.S. reports support for attending meetings/travel from Gilead and Abvvie. M.S. reports funding from Gilead and Abbvie for investigator-initiated research unrelated to this work; and consultant fees from Gilead Sciences for activities unrelated to this work. All other authors had nothing to declare.
Figures

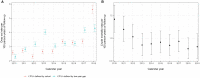
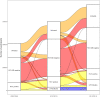
References
-
- Sogni P, Gilbert C, Lacombe K. et al. All-oral direct-acting antiviral regimens in HIV/hepatitis C virus–co-infected patients with cirrhosis are efficient and safe: real-life results from the prospective ANRS CO13–HEPAVIH cohort. Clin Infect Dis 2016;63:763–70. - PubMed
-
- World Health Organization. Interim Guidance for Country Validation of Viral Hepatitis Elimination. 2021. https://www.who.int/publications/i/item/9789240028395 (3 November 2023, date last accessed).
-
- Polaris Observatory HCV Collaborators. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol 2022;7:396–415. - PubMed
-
- Platt L, Easterbrook P, Gower E. et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016;16:797–808. - PubMed

