Real-world Cohort Study on the Effectiveness and Safety of Filgotinib Use in Ulcerative Colitis
- PMID: 38066679
- PMCID: PMC11760993
- DOI: 10.1093/ecco-jcc/jjad187
Real-world Cohort Study on the Effectiveness and Safety of Filgotinib Use in Ulcerative Colitis
Abstract
Background: Filgotinib is a small molecule with preferential inhibition of Janus kinase type 1, approved for the treatment of ulcerative colitis in Scotland in May 2022. We present the first real-world experience on its use in clinical practice.
Methods: In this retrospective, observational, cohort study we assessed patients with active ulcerative colitis who received filgotinib in NHS Lothian, Scotland. Baseline demographic, phenotype, and follow-up data were collected via review of electronic medical records.
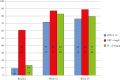
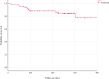
Results: We included 91 patients with median treatment duration of 39 weeks (interquartile range [IQR] 23-49). Among the cohort, 67% [61/91] were biologic- and small molecule-naïve, and 20.9% [19/91] had failed one and 12.1% [11/91] two or more classes of advanced therapy. Of the biologic- and small molecule-naïve patients, 18% [11/61] were also thiopurine-naïve. Clinical remission [partial Mayo score <2] was achieved in 71.9% [41/57] and 76.4% [42/55] of patients at Weeks 12 and 24 respectively. Biochemical remission [C reactive protein ≤5 mg/L] was achieved in 87.3% [62/71] at Week 12 and 88.9% [40/45] at Week 24. Faecal biomarker [calprotectin <250 µg/g] remission was achieved in 82.8% [48/58] at Week 12 and 79.5% [35/44] at Week 24. At the end of follow-up, median 42 weeks [IQR 27-50], 82.4% [75/91] of patients remained on filgotinib. Severe adverse events leading to drug discontinuation occurred in 2.2% [2/91] and there were 8.8% [8/91] moderate adverse events that required temporary discontinuation.
Conclusion: These are the first reported data on the real-world efficacy and safety of filgotinib in ulcerative colitis. Our findings demonstrate that filgotinib is an effective and low-risk treatment option for these patients.
Keywords: Ulcerative colitis; filgotinib; inflammatory bowel disease; small molecule.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
BG has served as consultant to Galapagos and Abbvie and as speaker for Abbvie, Jansen, Takeda, Pfizer, and Galapagos. NP has served as a speaker for Janssen, Takeda and Pfizer. CL has acted as a consultant to Abbvie, Janssen, Takeda, Pfizer, Galapagos, Bristol Myers Squibb, B.I., Sandoz, Novartis, GSK, Gilead, ViforPharma, Dr Falk, Trellus Health, and Iterative Scopes; he has received speaking fees and travel support from Pfizer, Janssen, Abbvie, Galapagos, MSD, Takeda, Shire, Ferring, Hospira, and Dr Falk. G-RJ has served as a speaker for Takeda, Janssen, Abbvie, Fresnius, and Ferring.
Figures
References
-
- Web Scottish Filgotinib Approval . 2022. https://www.scottishmedicines.org.uk/medicines-advice/filgotinib-jyselec... Accessed July 26, 2023.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials