Thrombotic anti-PF4 immune disorders: HIT, VITT, and beyond
- PMID: 38066843
- PMCID: PMC10727100
- DOI: 10.1182/hematology.2023000503
Thrombotic anti-PF4 immune disorders: HIT, VITT, and beyond
Abstract
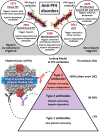
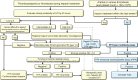
Antibodies against the chemokine platelet factor 4 (PF4) occur often, but only those that activate platelets induce severe prothrombotic disorders with associated thrombocytopenia. Heparin-induced thrombocytopenia (HIT) is the prototypic anti-PF4 disorder, mediated by strong activation of platelets through their FcγIIa (immunoglobulin G [IgG]) receptors (FcγRIIa). Concomitant pancellular activation (monocytes, neutrophils, endothelium) triggers thromboinflammation with a high risk for venous and arterial thrombosis. The classic concept of HIT is that anti-PF4/heparin IgG, recognizing antigen sites on (cationic) PF4 that form in the presence of (anionic) heparin, constitute the heparin-dependent antibodies that cause HIT. Accordingly, HIT is managed by anticoagulation with a nonheparin anticoagulant. In 2021, adenovirus vector COVID-19 vaccines triggered the rare adverse effect "vaccine-induced immune thrombotic thrombocytopenia" (VITT), also caused by anti-PF4 IgG. VITT is a predominantly heparin-independent platelet-activating disorder that requires both therapeutic-dose anticoagulation and inhibition of FcγRIIa-mediated platelet activation by high-dose intravenous immunoglobulin (IVIG). HIT and VITT antibodies bind to different epitopes on PF4; new immunoassays can differentiate between these distinct HIT-like and VITT-like antibodies. These studies indicate that (1) severe, atypical presentations of HIT ("autoimmune HIT") are associated with both HIT-like (heparin-dependent) and VITT-like (heparin-independent) anti-PF4 antibodies; (2) in some patients with severe acute (and sometimes chronic, recurrent) thrombosis, VITT-like antibodies can be identified independent of proximate heparin exposure or vaccination. We propose to classify anti-PF4 antibodies as type 1 (nonpathogenic, non- platelet activating), type 2 (heparin dependent, platelet activating), and type 3 (heparin independent, platelet activating). A key concept is that type 3 antibodies (autoimmune HIT, VITT) require anticoagulation plus an adjunct treatment, namely high-dose IVIG, to deescalate the severe anti-PF4 IgG-mediated hypercoagulability state.
Copyright © 2023 by The American Society of Hematology.
Conflict of interest statement
Andreas Greinacher: grants and nonfinancial support: Aspen, Boehringer Ingelheim, MSD, Bristol Myers Squibb, Paringenix, Bayer Healthcare, Gore Inc., Rovi, Sagent, Biomarin/Prosensa, Portola, Ergomed, GTH e.V.; personal fees: Aspen, Boehringer Ingelheim, MSD, Macopharma, Bristol Myers Squibb, Chromatec, Werfen (Instrumentation Laboratory).
Theodore E. Warkentin: honoraria: Alexion, Werfen (Instrumentation Laboratory); royalties: Informa (Taylor & Francis); consultancy: Aspen Canada, Aspen Global, CSL Behring, Ergomed, Paradigm Pharmaceuticals, Octapharma, Veralox Therapeutics; research funding: Werfen (Instrumentation Laboratory).
Figures


Similar articles
-
Platelet-activating anti-PF4 disorders: An overview.Semin Hematol. 2022 Apr;59(2):59-71. doi: 10.1053/j.seminhematol.2022.02.005. Epub 2022 Feb 20. Semin Hematol. 2022. PMID: 35512902 Review.
-
Laboratory Testing for Heparin-Induced Thrombocytopenia and Vaccine-Induced Immune Thrombotic Thrombocytopenia Antibodies: A Narrative Review.Semin Thromb Hemost. 2023 Sep;49(6):621-633. doi: 10.1055/s-0042-1758818. Epub 2022 Dec 1. Semin Thromb Hemost. 2023. PMID: 36455619 Free PMC article. Review.
-
Classification of Platelet-Activating Anti-Platelet Factor 4 Disorders.Int J Lab Hematol. 2025 May 13. doi: 10.1111/ijlh.14486. Online ahead of print. Int J Lab Hematol. 2025. PMID: 40358013 Review.
-
Therapeutic strategies in FcγIIA receptor-dependent thrombosis and thromboinflammation as seen in heparin-induced thrombocytopenia (HIT) and vaccine-induced immune thrombocytopenia and thrombosis (VITT).Expert Opin Pharmacother. 2024 Feb;25(3):281-294. doi: 10.1080/14656566.2024.2328241. Epub 2024 Mar 12. Expert Opin Pharmacother. 2024. PMID: 38465524 Review.
-
Unique features of vaccine-induced immune thrombotic thrombocytopenia; a new anti-platelet factor 4 antibody-mediated disorder.Int J Hematol. 2023 Mar;117(3):341-348. doi: 10.1007/s12185-022-03516-4. Epub 2022 Dec 27. Int J Hematol. 2023. PMID: 36574172 Free PMC article. Review.
Cited by
-
Autoimmune Heparin-Induced Thrombocytopenia.J Clin Med. 2023 Nov 3;12(21):6921. doi: 10.3390/jcm12216921. J Clin Med. 2023. PMID: 37959386 Free PMC article. Review.
-
Demystifying autoimmune HIT: what it is, when to test, and how to treat.Hematology Am Soc Hematol Educ Program. 2024 Dec 6;2024(1):403-408. doi: 10.1182/hematology.2024000565. Hematology Am Soc Hematol Educ Program. 2024. PMID: 39644061 Free PMC article. Review.
-
Endothelial cell activation enhances thromboinflammation in vaccine-induced immune thrombotic thrombocytopenia.Blood Adv. 2025 Jun 24;9(12):2891-2906. doi: 10.1182/bloodadvances.2024014165. Blood Adv. 2025. PMID: 40085945 Free PMC article.
-
VITT Pathophysiology: An Update.Vaccines (Basel). 2025 Jun 17;13(6):650. doi: 10.3390/vaccines13060650. Vaccines (Basel). 2025. PMID: 40573981 Free PMC article. Review.
-
Anti-PF4/ Heparin Antibodies Early Seroconversion in Hip Fracture Patients Receiving Low Molecular Weight Heparin Prophylaxis: a Pilot Study of 100 Consecutive Patients.Arch Bone Jt Surg. 2025;13(5):291-298. doi: 10.22038/ABJS.2025.80206.3661. Arch Bone Jt Surg. 2025. PMID: 40630816 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

