Clinical manifestations of telomere biology disorders in adults
- PMID: 38066848
- PMCID: PMC10726987
- DOI: 10.1182/hematology.2023000490
Clinical manifestations of telomere biology disorders in adults
Abstract
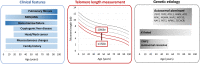
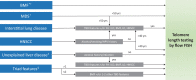
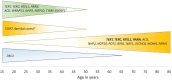
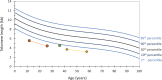
Telomere biology disorders (TBDs) are a spectrum of inherited bone marrow failure syndromes caused by impaired telomere function due to pathogenic germline variants in genes involved in telomere maintenance. TBDs can affect many organ systems and are often thought of as diseases of childhood. However, TBDs may present in mid- or even late adulthood with features similar to but not always the same as the childhood-onset TBDs. Adult-onset TBDs are often cryptic with isolated pulmonary, liver, or hematologic disease, or cancer, and may lack the classic disease-defining triad of abnormal skin pigmentation, nail dysplasia, and oral leukoplakia. Diagnostics include detection of very short leukocyte telomeres and germline genetic testing. Notably, adult-onset TBDs may show telomeres in the 1st to 10th percentile for age, and some cases may not have an identifiable genetic cause. TBD genetic etiology includes all modes of inheritance, with autosomal dominant the most frequent in adult-onset disease. Variable symptom onset due to incomplete penetrance, variable expressivity, and genetic anticipation add to the diagnostic challenges. Adult-onset TBDs are likely underrecognized, but their correct identification is of utmost importance, since affected patients are faced with numerous clinical complications, including but not limited to an increased risk of malignancies requiring close surveillance for early detection. Currently lung, liver, or hematopoietic cell transplants are the only curative therapeutic approaches but can be complicated by comorbidities, despite improved medical care. This review highlights the challenges of identifying adult-onset TBDs and addresses currently recommended clinical screening measures and therapy options.
Copyright © 2023 by The American Society of Hematology.
Conflict of interest statement
Marena R. Niewisch: no competing financial interests to declare.
Fabian Beier: no competing financial interests to declare.
Sharon A. Savage: no competing financial interests to declare.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

