Langerhans cell histiocytosis: promises and caveats of targeted therapies in high-risk and CNS disease
- PMID: 38066856
- PMCID: PMC10726990
- DOI: 10.1182/hematology.2023000439
Langerhans cell histiocytosis: promises and caveats of targeted therapies in high-risk and CNS disease
Abstract
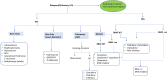
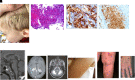
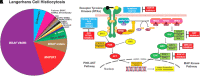
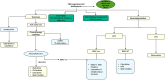
Langerhans cell histiocytosis (LCH) is a rare myeloid neoplasm driven by activating mutations in the MAPK pathway, most commonly BRAF-V600E and MAP2K1. It affects children and adults, with a wide spectrum of clinical presentations ranging from self-limited to multisystem (MS) life-threatening forms. LCH is defined by the accumulation of CD1a+/CD207+ cells in different organs, and patients with liver, spleen, or hematopoietic system involvement have a higher risk of mortality. Patients with neurodegeneration (ND) have devastating outcomes and are resistant to systemic therapies. MS-LCH is treated with risk-adapted therapy, but many patients require multiple salvage regimens that are myelosuppressive and expensive. MAPK inhibitors are increasingly being used, but most patients relapse upon discontinuation of therapy. Here, we review the management of central nervous system disease and how novel cerebrospinal fluid biomarkers might predict patients at high risk of ND who could benefit from early MAPK inhibition. Further, we discuss treatment strategies for refractory/relapsed (R/R) LCH, with a focus on MAPK inhibitors' efficacy and challenges (ie, the unknown): long-term toxicity in children, optimal duration, if they are curative, whether it is safe to combine them with chemotherapy, and their high price tag. Lastly, emerging strategies, such as the new panRAF inhibitor (Day 101) in patients with R/R LCH, ERK1/2 or CSF1R inhibition in patients with MEK1/2 inhibitor resistance, and targeting the microenvironment (checkpoint plus MEK inhibition) or senescent cells (mTOR or BCL-XL inhibitors) in R/R patients, are also examined.
Copyright © 2023 by The American Society of Hematology.
Conflict of interest statement
Oussama Abla: no competing financial interests to declare.
Figures
References
-
- Diamond EL, Durham BH, Haroche J, et al.. Diverse and targetable kinase alterations drive histiocytic neoplasms. Cancer Discov. 2016;6(2):154-165. doi:10.1158/2159-8290.CD-15-0913. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

