Next-generation therapy for lower-risk MDS
- PMID: 38066862
- PMCID: PMC10727062
- DOI: 10.1182/hematology.2023000520
Next-generation therapy for lower-risk MDS
Abstract
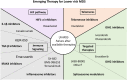
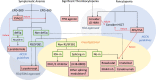
Myelodysplastic syndromes (MDS) are malignant myeloid neoplasms characterized by ineffective clonal hematopoiesis leading to peripheral blood cytopenia and a variable risk of transformation to acute myeloid leukemia. In lower-risk (LR) MDS, as defined by prognostic scoring systems recently updated with the addition of a mutation profile, therapeutic options aim to reduce cytopenia, mainly anemia. Although options for reducing the transfusion burden have recently been improved, erythropoiesis-stimulating agents (ESAs), lenalidomide, hypomethylating agents, and, more recently, luspatercept have shown efficacy in rarely more than 50% of patients with a duration of response often far inferior to the patient's life expectancy. Nevertheless, several new therapies are currently under investigation aiming at improving cytopenia in patients with LR-MDS, mostly by targeting different biological pathways. Targeting ligands of the transforming growth factor β pathway has led to the approval of luspatercept in LR-MDS with ring sideroblasts or SF3B1 mutation, potentially replacing first-line ESAs in this population. Here, we also discuss the evolving standard of care for the treatment of LR-MDS and explore some of the most promising next-generation agents under investigation.
Copyright © 2023 by The American Society of Hematology.
Conflict of interest statement
Marie Sébert: honoraria: Abbvie, Servier, BMS, Gilead, Jazz Pharmaceuticals.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous