Prevention, diagnosis, and management of PE and DVT in pregnant women
- PMID: 38066865
- PMCID: PMC10727078
- DOI: 10.1182/hematology.2023000476
Prevention, diagnosis, and management of PE and DVT in pregnant women
Abstract
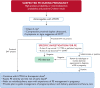
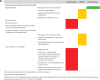
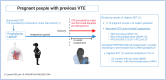
Venous thromboembolism (VTE) is a leading cause of maternal morbidity and mortality worldwide. Despite the impact of VTE on pregnant and postpartum people and on society, guidelines addressing prevention, diagnosis, and management of VTE in pregnant and postpartum people frequently are based on recommendations from expert opinion and are extrapolated from data in nonpregnant populations. Pregnant individuals are frequently excluded from clinical trials, which is a barrier to providing safe, effective care. Anchoring to a case discussion, this review provides an update on recently published and ongoing randomized clinical trials (RCTs), prospective clinical management studies, and other research in this area. It highlights, in particular, the results of the Highlow RCT, which addresses optimal prevention of recurrence during pregnancy in people with prior VTE. Finally, we raise awareness of the impact of national and international clinical trial networks on the conduct of RCTs in pregnancy. We conclude, based on these data, that academic VTE clinical trials in pregnant women can and must be done.
Copyright © 2023 by The American Society of Hematology.
Conflict of interest statement
Barry Kevane reports honoraria unrelated to this project from Bayer (advisory board membership) and Leo Pharma.
Fionnuala Ní Áinle reports grants for investigator-initiated studies paid to her institution and unrelated to this project from Sanofi, Daiichi Sankyo, and Bayer and acting as a consultant (membership of a trial executive committee, paid to institution) for Boston Scientific.
Figures




References
-
- Abdul Sultan A, Tata LJ, Grainge MJ, West J. The incidence of first venous thromboembolism in and around pregnancy using linked primary and secondary care data: a population based cohort study from England and comparative meta-analysis. PLoS One. 2013;8(7):e70310. doi:10.1371/journal.pone.0070310. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

