Checkpoint inhibitors
- PMID: 38066867
- PMCID: PMC10727098
- DOI: 10.1182/hematology.2023000523
Checkpoint inhibitors
Abstract
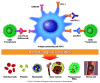
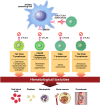
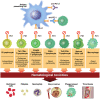
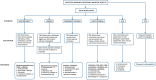
Immune checkpoint inhibitors are a class of antineoplastic therapies that unleash immune cells to kill malignant cells. These medications commonly cause immune-related adverse effects due to activated adaptive and innate immune cells, autoantibody production, and/or cytokine dysregulation. Hematologic toxicities are rare and of uncertain mechanism, and therefore management is often based on experiences with familiar conditions involving these perturbed immune responses. Management is challenging because one must attend to the hematologic toxicity while simultaneously attending to the malignancy, with the imperative that therapeutic effects be maintained or minimally interrupted when possible.
Copyright © 2023 by The American Society of Hematology.
Conflict of interest statement
Michael H. Kroll: no competing financial interests to declare.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

